Difference between revisions of "GCSE Chemistry Required Practical: Concentration and Rate of Reaction"
| Line 10: | Line 10: | ||
|} | |} | ||
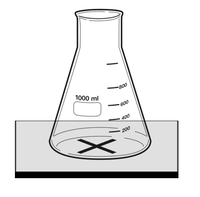
#Draw a large thick black cross on to a [[White Tile]] and place a 100ml [[Conical Flask]] on top as shown in the [[diagram]]. | #Draw a large thick black cross on to a [[White Tile]] and place a 100ml [[Conical Flask]] on top as shown in the [[diagram]]. | ||
| − | #Measure 10ml of 0.25[[Molarity|M]] | + | #Measure 10ml of 0.25[[Molarity|M]] Sodium Thiosulphate using a [[Measuring Cylinder]] and add to the [[Conical Flask]]. |
#Measure 40ml of [[Water]] and add to the [[Conical Flask]]. | #Measure 40ml of [[Water]] and add to the [[Conical Flask]]. | ||
#Measure 10ml of 2[[Molarity|M]] [[Hydrochloric Acid]] using a [[Measuring Cylinder]]. | #Measure 10ml of 2[[Molarity|M]] [[Hydrochloric Acid]] using a [[Measuring Cylinder]]. | ||
#Add the 10ml of [[Hydrochloric Acid]] to the [[Conical Flask]] while starting a [[stopwatch]]. | #Add the 10ml of [[Hydrochloric Acid]] to the [[Conical Flask]] while starting a [[stopwatch]]. | ||
#Observe the cross from above, through the [[solution]]. When the cross is no longer visible through the [[solution]] stop the [[stopwatch]] and record the [[time]] in [[second]]s. | #Observe the cross from above, through the [[solution]]. When the cross is no longer visible through the [[solution]] stop the [[stopwatch]] and record the [[time]] in [[second]]s. | ||
| − | #Repeat the [[experiment]] with 20ml | + | #Repeat the [[experiment]] with 20ml Sodium Thiosulphate and 30ml [[Water]], 30ml Sodium Thiosulphate and 20ml [[Water]], 40ml Sodium Thiosulphate and 10ml [[Water]] and 50ml Sodium Thiosulphate. |
| − | #Repeat the same [[experiment]] for all [[ratio]]s of | + | #Repeat the same [[experiment]] for all [[ratio]]s of Sodium Thiosulphate and [[Water]] a further two times to calculate an [[Mean Average|average]]. |
====Observation of Rate by Gas Produced==== | ====Observation of Rate by Gas Produced==== | ||
Revision as of 19:33, 8 April 2019
Contents
Key Stage 4
Meaning
Investigate how concentration affects the rate of reaction.
Method
Observation of Rate by Opacity
- Draw a large thick black cross on to a White Tile and place a 100ml Conical Flask on top as shown in the diagram.
- Measure 10ml of 0.25M Sodium Thiosulphate using a Measuring Cylinder and add to the Conical Flask.
- Measure 40ml of Water and add to the Conical Flask.
- Measure 10ml of 2M Hydrochloric Acid using a Measuring Cylinder.
- Add the 10ml of Hydrochloric Acid to the Conical Flask while starting a stopwatch.
- Observe the cross from above, through the solution. When the cross is no longer visible through the solution stop the stopwatch and record the time in seconds.
- Repeat the experiment with 20ml Sodium Thiosulphate and 30ml Water, 30ml Sodium Thiosulphate and 20ml Water, 40ml Sodium Thiosulphate and 10ml Water and 50ml Sodium Thiosulphate.
- Repeat the same experiment for all ratios of Sodium Thiosulphate and Water a further two times to calculate an average.
Observation of Rate by Gas Produced
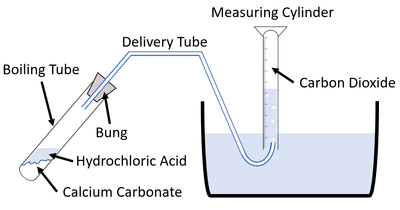
| This diagram shows a possible setup for measuring the gas given off during an experiment. |
- Set up the apparatus as shown in the diagram.
- Measure 10ml 2M Hydrochloric Acid using a Measuring Cylinder.
- Add the Hydrochloric Acid to the Boiling Tube.
- Add 1 spatula of Marble chips Calcium Carbonate to the Boiling Tube.
- Attach the bung.
- Fill the 50ml Measuring Cylinder with water in the tub and hold it over the end of the delivery tube to collect the bubbles.
- Start a stopwatch and record the volume of gas in the Measuring Cylinder every 10 seconds.
- Repeat this experiment with 10ml 1M Hydrochloric Acid.