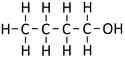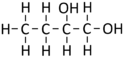Difference between revisions of "Butanol"
| Line 28: | Line 28: | ||
|[[File:BallandStickButan-1-ol.png|center|125px]] | |[[File:BallandStickButan-1-ol.png|center|125px]] | ||
|} | |} | ||
| + | |||
| + | : The [[combustion]] of [[Butanol]] [[product|produces]] [[Carbon Dioxide]] and [[Water]]. | ||
| + | : [[Butanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| + | : <chem>C4H9OH + 6O2 -> 4CO2 + 5H2O</chem> | ||
| + | : [[Butanol]] can also be [[oxidise]]d at low [[temperature]]s to [[product|produce]] [[Water]] and the [[Carboxylic Acid]]; [[Butanoic Acid]]. | ||
| + | : <chem>C4H9OH + O2 -> C3H7COOH + H2O</chem> | ||
: [[Butanol]] can be [[oxidise]]d to [[product|produce]] [[Carbon Dioxide]] and [[Water]]. | : [[Butanol]] can be [[oxidise]]d to [[product|produce]] [[Carbon Dioxide]] and [[Water]]. | ||
: [[Butanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | : [[Butanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| − | |||
====Butan-12-diol==== | ====Butan-12-diol==== | ||
| Line 47: | Line 52: | ||
|} | |} | ||
| − | : '''Butandiol''' | + | : The [[combustion]] of '''Butandiol''' [[product|produces]] [[Carbon Dioxide]] and [[Water]]. |
: '''Butandiol''' + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | : '''Butandiol''' + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
: <chem>2C4H8(OH)2 + 11O2 -> 8CO2 + 10H2O</chem> | : <chem>2C4H8(OH)2 + 11O2 -> 8CO2 + 10H2O</chem> | ||
Revision as of 07:21, 24 April 2019
Contents
Key Stage 3
Meaning
Butanol is a compound with chemical formula C4H9OH.
About Butanol
- Butanol is a transparent liquid (at room temperature).
- Butanol can be oxidised to produce Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Butanol is an alcohol molecule with chemical formula C4H9OH.
About Butanol
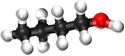
Butan-1-ol
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C4H9OH | CH3CH2CH2CH2OH |
- The combustion of Butanol produces Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
- <chem>C4H9OH + 6O2 -> 4CO2 + 5H2O</chem>
- Butanol can also be oxidised at low temperatures to produce Water and the Carboxylic Acid; Butanoic Acid.
- <chem>C4H9OH + O2 -> C3H7COOH + H2O</chem>
- Butanol can be oxidised to produce Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
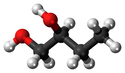
Butan-12-diol
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C4H8(OH)2 | CH3CH2CHOHCH2OH |
- The combustion of Butandiol produces Carbon Dioxide and Water.
- Butandiol + Oxygen → Carbon Dioxide + Water
- <chem>2C4H8(OH)2 + 11O2 -> 8CO2 + 10H2O</chem>