Difference between revisions of "PH"
(→Key Stage 4) |
|||
| Line 2: | Line 2: | ||
===Meaning=== | ===Meaning=== | ||
[[File:pHScale.png|right|300px|thumb|A '''pH''' scale with the colours of [[Universal Indicator]] at those '''pH''' values and some examples of [[substance]]s at those '''pH''' values.]] | [[File:pHScale.png|right|300px|thumb|A '''pH''' scale with the colours of [[Universal Indicator]] at those '''pH''' values and some examples of [[substance]]s at those '''pH''' values.]] | ||
| − | The '''pH''' scale is a numbered list from | + | The '''pH''' scale is a numbered list from 0 to 14 that is used to identify how [[Acid|acidic]] or [[Base|basic]] a [[substance]] is. |
===About pH=== | ===About pH=== | ||
: '''pH''' is written with a lower case p and an upper case H and refers to the 'power of Hydrogen' as there are free [[Hydrogen]] [[ion]]s in an [[acid]]. | : '''pH''' is written with a lower case p and an upper case H and refers to the 'power of Hydrogen' as there are free [[Hydrogen]] [[ion]]s in an [[acid]]. | ||
| − | : [[Acid]]s | + | : [[Acid]]s have a '''pH''' of less than 7 with the strongest [[acid]]s being at '''pH''' 0. |
| − | : [[Base]]s | + | : [[Base]]s have a '''pH''' greater than 7 with the strongest [[base]]s being at '''pH''' 14. Remember [[alkali]]s are a [[base]] [[dissolve]]d in [[water]]. |
: '''pH''' 7.0 is [[Neutral (Chemistry)|neutral]]. | : '''pH''' 7.0 is [[Neutral (Chemistry)|neutral]]. | ||
| − | ==Key Stage 4== | + | ==Key Stage 4 Foundation== |
===Meaning=== | ===Meaning=== | ||
| − | The '''pH''' scale is a numbered list from | + | The '''pH''' scale is a numbered list from 0 to 14 that is used to identify how [[Acid|acidic]] or [[Base|basic]] a [[substance]] is. |
===About the pH Scale=== | ===About the pH Scale=== | ||
| − | |||
: The '''pH''' of a [[solution]] is determined by the strength and [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] in an [[acid]] or [[Hydroxide Ion (Chemistry)|Hydroxide ions]] in an [[alkali]]. | : The '''pH''' of a [[solution]] is determined by the strength and [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] in an [[acid]] or [[Hydroxide Ion (Chemistry)|Hydroxide ions]] in an [[alkali]]. | ||
| + | : An [[acid]] will have a greater [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] the lower on the '''pH''' scale. | ||
| + | : An [[alkali]] will have a greater [[concentration]] of [[Hydroxide Ion (Chemistry)|Hydroxide ions]] the higher on the '''pH''' scale. | ||
| + | : A [[Neutral (Chemistry)|neutral]] [[solution]] or [[pure]] [[water]] will have an equal [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] and [[Hydroxide Ion (Chemistry)|Hydroxide ions]] and have a '''pH''' of 7. | ||
| − | ==== | + | ==Key Stage 4 Higher== |
| + | ===Meaning=== | ||
| + | The '''pH''' scale is a numbered list from 0 to 14 that is used to identify how [[Acid|acidic]] or [[Base|basic]] a [[substance]] is. | ||
| + | |||
| + | ===About the pH Scale=== | ||
| + | : The '''pH''' of a [[solution]] is determined by the strength and [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] in an [[acid]] or [[Hydroxide Ion (Chemistry)|Hydroxide ions]] in an [[alkali]]. | ||
| + | : An [[acid]] will have a greater [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] the lower on the '''pH''' scale. | ||
| + | : An [[alkali]] will have a greater [[concentration]] of [[Hydroxide Ion (Chemistry)|Hydroxide ions]] the higher on the '''pH''' scale. | ||
| + | : A [[Neutral (Chemistry)|neutral]] [[solution]] or [[pure]] [[water]] will have an equal [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] and [[Hydroxide Ion (Chemistry)|Hydroxide ions]] and have a '''pH''' of 7. | ||
: In [[acid]]s for a '''pH''' decrease of 1 there must be 10 times greater [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] in [[solution]]. | : In [[acid]]s for a '''pH''' decrease of 1 there must be 10 times greater [[concentration]] of [[Hydrogen Ion (Chemistry)|Hydrogen ions]] in [[solution]]. | ||
Revision as of 17:12, 28 June 2019
Contents
Key Stage 3
Meaning
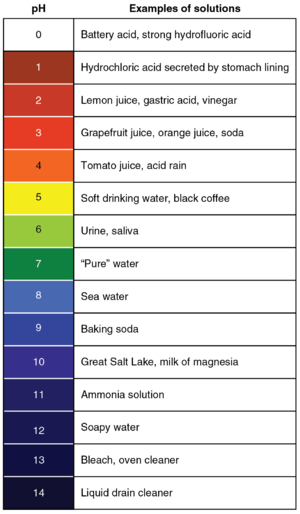
A pH scale with the colours of Universal Indicator at those pH values and some examples of substances at those pH values.
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About pH
- pH is written with a lower case p and an upper case H and refers to the 'power of Hydrogen' as there are free Hydrogen ions in an acid.
- Acids have a pH of less than 7 with the strongest acids being at pH 0.
- Bases have a pH greater than 7 with the strongest bases being at pH 14. Remember alkalis are a base dissolved in water.
- pH 7.0 is neutral.
Key Stage 4 Foundation
Meaning
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About the pH Scale
- The pH of a solution is determined by the strength and concentration of Hydrogen ions in an acid or Hydroxide ions in an alkali.
- An acid will have a greater concentration of Hydrogen ions the lower on the pH scale.
- An alkali will have a greater concentration of Hydroxide ions the higher on the pH scale.
- A neutral solution or pure water will have an equal concentration of Hydrogen ions and Hydroxide ions and have a pH of 7.
Key Stage 4 Higher
Meaning
The pH scale is a numbered list from 0 to 14 that is used to identify how acidic or basic a substance is.
About the pH Scale
- The pH of a solution is determined by the strength and concentration of Hydrogen ions in an acid or Hydroxide ions in an alkali.
- An acid will have a greater concentration of Hydrogen ions the lower on the pH scale.
- An alkali will have a greater concentration of Hydroxide ions the higher on the pH scale.
- A neutral solution or pure water will have an equal concentration of Hydrogen ions and Hydroxide ions and have a pH of 7.
- In acids for a pH decrease of 1 there must be 10 times greater concentration of Hydrogen ions in solution.