Difference between revisions of "Activation Energy"
(Created page with "==Key Stage 4== ===Meaning=== '''Activation energy''' is the amount of energy needed to begin a chemical reaction. ===About Activation Energy=== ===...") |
|||
| Line 22: | Line 22: | ||
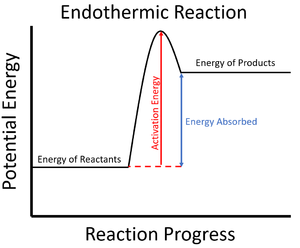
| style="height:20px; width:200px; text-align:center;" |The '''activation energy''' is shown in this [[Reaction Profile|reaction profile]] for an [[endothermic]] [[Chemical Reaction|reaction]]. | | style="height:20px; width:200px; text-align:center;" |The '''activation energy''' is shown in this [[Reaction Profile|reaction profile]] for an [[endothermic]] [[Chemical Reaction|reaction]]. | ||
|} | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Activation energy, GCSE Chemistry; Student Book, Collins, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Activation energy, page 233, GCSE Combined Science Trilogy 1, Hodder, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Activation energy, pages 117, 130, 136, GCSE Chemistry; Third Edition, Oxford University Press, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Activation energy, pages 122, GCSE Combined Science Trilogy 2, Hodder, AQA '] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Activation energy, pages 131, 151, GCSE Chemistry, Hodder, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09656 ''Activation energy, pages 136, 138, GCSE Combined Science; The Revision Guide, CGP, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Activation energy, pages 156, 166, 167, GCSE Combined Science Trilogy; Chemistry, CGP, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Activation energy, pages 181, 198, 199, GCSE Chemistry, CGP, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d30 ''Activation energy, pages 62, 67, GCSE Chemistry; The Revision Guide, CGP, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Activation energy; and catalyst, page 153, GCSE Chemistry, Hodder, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Activation energy; And catalysts, pages 124, GCSE Combined Science Trilogy 2, Hodder, AQA '] | ||
Revision as of 17:47, 27 October 2019
Contents
Key Stage 4
Meaning
Activation energy is the amount of energy needed to begin a chemical reaction.
About Activation Energy
Foundation
- Chemical reactions can't take place unless there is enough energy to cause the reactant molecules to collide and react.
- Different reactions need different amount of activation energy to start.
- The activation energy can be reached by heating the mixture of reactants until they have enough energy to react.
- Sometimes reactants at room temperature have enough energy to react. If the mixture of reactants was lower than room temperature they might not have enough energy to begin reacting.
- Once the activation energy is reached the reaction will continue as energy is released when chemical bonds form to make the products.
Higher
- Activation energy is needed to allow the reactant molecules to collide with one another hard enough to break the bonds holding them together. This allows new bonds to form making the product.
Examples
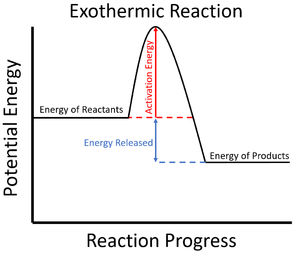
| The activation energy is shown in this reaction profile for an exothermic reaction. | The activation energy is shown in this reaction profile for an endothermic reaction. |
References
AQA
- Activation energy, GCSE Chemistry; Student Book, Collins, AQA'
- Activation energy, page 233, GCSE Combined Science Trilogy 1, Hodder, AQA'
- Activation energy, pages 117, 130, 136, GCSE Chemistry; Third Edition, Oxford University Press, AQA'
- Activation energy, pages 122, GCSE Combined Science Trilogy 2, Hodder, AQA '
- Activation energy, pages 131, 151, GCSE Chemistry, Hodder, AQA'
- Activation energy, pages 136, 138, GCSE Combined Science; The Revision Guide, CGP, AQA'
- Activation energy, pages 156, 166, 167, GCSE Combined Science Trilogy; Chemistry, CGP, AQA'
- Activation energy, pages 181, 198, 199, GCSE Chemistry, CGP, AQA'
- Activation energy, pages 62, 67, GCSE Chemistry; The Revision Guide, CGP, AQA'
- Activation energy; and catalyst, page 153, GCSE Chemistry, Hodder, AQA'
- Activation energy; And catalysts, pages 124, GCSE Combined Science Trilogy 2, Hodder, AQA '