Difference between revisions of "Empirical Formula"
| Line 175: | Line 175: | ||
:[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Formula, formulae; empirical, pages 62-3, 90-1, 252, GCSE Chemistry; Student Book, Collins, AQA ''] | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Formula, formulae; empirical, pages 62-3, 90-1, 252, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Empirical formula, page 217, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Empirical formulae, page 73, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Empirical formulae, pages 71, 72, 77, 78, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Empirical formulas, pages 27, 30, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Empirical formulas, pages 90, 93, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
Revision as of 13:36, 19 November 2019
Contents
Key Stage 4
Meaning
An empirical formula is the simplest ratio of the different types of atom in a compound.
About Empirical Formulae
The empirical formula of a compound may not be the same as the chemical formula:
- Ethane - Chemical Formula C2H6, Empirical Formula CH3
- Ethene - Chemical Formula C2H4, Empirical Formula CH2
- Propene - Chemical Formula C3H6, Empirical Formula CH2
- Glucose - Chemical Formula C6H12O6, Empirical Formula CH2O
- Lactic Acid - Chemical Formula C3H6O3, Empirical Formula CH2O
- Empirical formulae are calculated from the amount of atoms in a chemical reaction.
- The number of atoms can be found if you know the mass of different elements and the relative atomic mass of the elements in the reaction.
Finding the Empirical Formula
| Number of Atoms | Skeletal Diagram | Ball and Stick Model |
| 100 atoms of Hydrogen react completely with 50 atoms of Oxygen. | ||
|
The ratio of Hydrogen atoms to Oxygen atoms H:O 100:50 2:1 So the empirical formula is H2O |
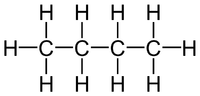
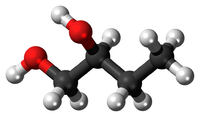
In this diagram there are 4 Carbon atoms, 10 Hydrogen atoms. C:H 4:10 2:5 So the empirical formula is C2H5 |
In this diagram there are 4 Carbon atoms, 10 Hydrogen atoms and 2 Oxygen atoms. C:H:O 4:10:2 2:5:1 So the empirical formula is C2H5O |
| 64g of Oxygen is found to react completely with 8g of Hydrogen. Find the empirical formula for the product. | 60g of Carbon is found to react completely with 160g of Oxygen. Find the empirical formula for the product. | 416g of Sulphur is found to react completely with Oxygen to produce 832g of product. Find the empirical formula for the product. |
| State the mass of all reactants. | State the mass of all reactants. | State the mass of all reactants.
|
| Find the number of moles of each element.
Number of Moles = (Mass/Relative Atomic Mass) Relative Atomic Mass of Oxygen = 16g
|
Find the number of moles of each element.
Number of Moles = (Mass/Relative Atomic Mass) Relative Atomic Mass of Carbon = 12g
|
Find the number of moles of each element.
Number of Moles = (Mass/Relative Atomic Mass) Relative Atomic Mass of Sulphur = 32g
|
| Find the Ratio:
8:4 2:1 |
Find the Ratio:
5:10 1:2 |
Find the Ratio:
13:26 1:2 |
| The empirical formula of the product is H2O | The empirical formula of the product is CO2 | The empirical formula of the product is SO2 |
References
AQA
Edexcel
- Empirical formula, page 217, GCSE Combined Science, Pearson Edexcel
- Empirical formulae, page 73, GCSE Chemistry, Pearson, Edexcel
- Empirical formulae, pages 71, 72, 77, 78, GCSE Chemistry, CGP, Edexcel
- Empirical formulas, pages 27, 30, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Empirical formulas, pages 90, 93, GCSE Combined Science; The Revision Guide, CGP, Edexcel