Difference between revisions of "Bond Energy"
| (One intermediate revision by one other user not shown) | |||
| Line 112: | Line 112: | ||
:[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Bond energy, page 147, GCSE Chemistry, Pearson, Edexcel ''] | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Bond energy, page 147, GCSE Chemistry, Pearson, Edexcel ''] | ||
:[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Bond energy, page 261, GCSE Combined Science, Pearson Edexcel ''] | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Bond energy, page 261, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Bonds; energy calculations, page 147, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Bonds; energy calculations, page 261, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Bond energies, page 42, GCSE Chemistry; The Revision Guide, CGP, OCR Gateway ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Bond energies, pages 106-107, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 11:22, 1 December 2019
Contents
Key Stage 4 Higher
Meaning
Bond Energy is the energy needed to break a chemical bond between two atoms.
About Bond Energy
- When chemical bonds are formed energy is released to the surroundings increasing the temperature. An exothermic process]].
- To break chemical bonds energy is needed to separate the atoms, decreasing the temperature of the surroundings. An endothermic process]].
Examples
Some common bond energies are given in the table below.
| Bond | Energy in kJ/mol |
| H-H | 436 |
| O=O | 498 |
| N≡N | 941 |
| C-C | 347 |
| C=C | 614 |
| C≡C | 839 |
| C-H | 413 |
| O-H | 464 |
| C=O | 799 |
| Cl-Cl | 243 |
| H-Cl | 432 |
| N-H | 391 |
These can be used to calculate the energy released per mole in a chemical reaction.
Example 1
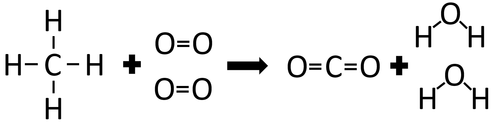
| In the reaction between Methane and Oxygen the chemical bonds in the reactants must be broken first before the bonds in the products are formed. |
There are 4 C-H bonds and 2 O=O bonds.
4 x 413 + 2 x 498 = 2648kJ
Therefore 2648kJ/mol are needed to break the bonds in the reactants.
There are 2 C=O bonds and 4 O-H bonds.
2 x 799 + 4 x 464 = 3454kJ
Therefore 3454kJ/mol is released when the bonds in the products form.
Once the reaction is complete 806kJ will be released.
Example 2
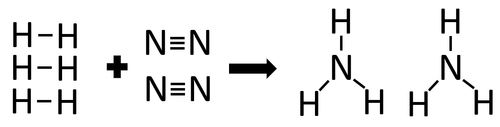
| In the reaction between Hydrogen and Nitrogen the chemical bonds in the reactants must be broken first before the bonds in the products are formed. |
There are 3 H-H bonds and 1 N≡N bonds.
3 x 436 + 1 x 941 = 2249kJ
Therefore 2249kJ/mol are needed to break the bonds in the reactants.
There are 6 N-H bonds.
6 x 391 = 2346kJ
Therefore 2346kJ/mol is released when the bonds in the products form.
Once the reaction is complete 97kJ will be released.
References
AQA
- Bond energies, page 63, GCSE Chemistry; The Revision Guide, CGP, AQA
- Bond energies, pages 133-5, GCSE Chemistry, Hodder, AQA
- Bond energies, pages 158, 159, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Bond energies, pages 183, 184, GCSE Chemistry, CGP, AQA
- Bond energies, pages 235-7, GCSE Combined Science Trilogy 1, Hodder, AQA
- Bond energy, pages 117-119, GCSE Chemistry; Third Edition, Oxford University Press, AQA
Edexcel
- Bond energies, page 136, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Bond energies, page 85, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Bond energies, pages 248-250, GCSE Chemistry, CGP, Edexcel
- Bond energy, page 147, GCSE Chemistry, Pearson, Edexcel
- Bond energy, page 261, GCSE Combined Science, Pearson Edexcel
- Bonds; energy calculations, page 147, GCSE Chemistry, Pearson, Edexcel
- Bonds; energy calculations, page 261, GCSE Combined Science, Pearson Edexcel