Difference between revisions of "Half Life"
(→Finding Half Life and Count Rate) |
|||
| (26 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | ==Key Stage 4== | + | ==Key Stage 4 Foundation== |
===Meaning=== | ===Meaning=== | ||
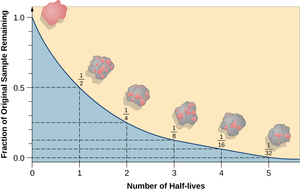
| − | [[File:HalfLifeGraph1.png|right|300px|thumb|A [[graph]] showing the | + | [[File:HalfLifeGraph1.png|right|300px|thumb|A [[graph]] showing the fraction of [[Unstable Isotope|unstable isotope]] left compared to the '''half life'''.]] |
A '''half life''' is the time it takes for half of the [[Unstable Isotope|unstable isotopes]] in a [[radioactive]] sample to [[Radioactive Decay|decay]]. | A '''half life''' is the time it takes for half of the [[Unstable Isotope|unstable isotopes]] in a [[radioactive]] sample to [[Radioactive Decay|decay]]. | ||
| Line 19: | Line 19: | ||
: The [[Count Rate|count rate]] of a sample of [[Unstable Isotope|unstable isotope]] can be predicted given its initial [[Count Rate|count rate]], its '''half life''' and the [[time]] it has been given. | : The [[Count Rate|count rate]] of a sample of [[Unstable Isotope|unstable isotope]] can be predicted given its initial [[Count Rate|count rate]], its '''half life''' and the [[time]] it has been given. | ||
| − | ===Finding Half Life | + | ===Finding Half Life from a Graph=== |
{| class="wikitable" | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |Find the '''half life''' from this [[graph]]. | ||
| + | | style="height:20px; width:300px; text-align:center;" |Find the '''half life''' from this [[graph]]. | ||
| + | |- | ||
| + | |[[File:HalfLifeGraph2.png|center|300px]] | ||
| + | |[[File:HalfLifeGraph3.png|center|300px]] | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |There is 100% of the [[isotope]] at the beginning. Draw a horizontal line from 50% to the curve. Draw a vertical line down from the curve to indicate the '''half life'''. | ||
| + | | style="height:20px; width:300px; text-align:center;" |The [[Count Rate|count rate]] began at 70,000Bq. Draw a horizontal line from 35,000 (half the initial count rate) to the curve. Draw a vertical line down from the curve to indicate the '''half life'''. | ||
| + | |- | ||
| + | |[[File:HalfLifeGraph2b.png|center|300px]] | ||
| + | |[[File:HalfLifeGraph3b.png|center|300px]] | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" | | ||
| + | '''Half Life''' = 4 [[minute]]s | ||
| + | | style="height:20px; width:300px; text-align:center;" | | ||
| + | '''Half Life''' = 6 [[hour]]s | ||
| + | |} | ||
| + | |||
| + | ===Calculating Half Life=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |An [[isotope]] had a [[Count Rate|count rate]] of 200Bq and 90 days later has a [[Count Rate|count rate]] of 25. Calculate the '''half life''' of this [[isotope]]. | ||
| + | | style="height:20px; width:300px; text-align:center;" |A [[radioactive]] [[sample]] begins with 224 [[atom]]s of an [[Unstable Isotope|unstable isotope]] 18 [[minute]]s later there are only 14 [[atom]]s of the [[Unstable Isotope|unstable isotope]] left. Calculate the '''half life''' of this [[isotope]]. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''1. Identify how many times the [[Count Rate|count rate]] has halved.''' | ||
| + | |||
| + | <math>200 ÷ 2 = 100</math> | ||
| + | |||
| + | <math>100 ÷ 2 = 50</math> | ||
| + | |||
| + | <math>50 ÷ 2 = 25</math> | ||
| + | |||
| + | The [[Count Rate|count rate]] has halved 3 times. | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''1. Identify how many times the number of [[Unstable Isotope|unstable]] [[atom]]s has halved.''' | ||
| + | |||
| + | <math>224 ÷ 2 = 112</math> | ||
| + | |||
| + | <math>112 ÷ 2 = 56</math> | ||
| + | |||
| + | <math>56 ÷ 2 = 28</math> | ||
| + | |||
| + | <math>56 ÷ 2 = 28</math> | ||
| + | |||
| + | The number of [[Unstable Isotope|unstable]] [[atom]]s has halved 4 times. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''2. Divide the time taken by the number of times the [[Count Rate|count rate]] has halved.''' | ||
| + | |||
| + | <math>90 ÷ 3 = 30</math> | ||
| + | |||
| + | '''Half Life''' = 30 days. | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''2. Divide the time taken by the number of times the number of [[Unstable Isotope|unstable]] [[atom]]s has halved.''' | ||
| + | |||
| + | <math>18 ÷ 4 = 4.5</math> | ||
| + | |||
| + | '''Half Life''' = 4.5 [[minute]]s | ||
| + | |} | ||
| + | |||
| + | ===Finding Half Life and Count Rate from a Table=== | ||
| + | {| border="1" style="border-collapse:collapse" | ||
| style="height:20px; width:100px; text-align:center;" |Time /s | | style="height:20px; width:100px; text-align:center;" |Time /s | ||
| style="height:20px; width:50px; text-align:center;" |0 | | style="height:20px; width:50px; text-align:center;" |0 | ||
| Line 49: | Line 109: | ||
:1000/2 = 500Bq | :1000/2 = 500Bq | ||
| − | :The [[isotope]] took 20 | + | :The [[isotope]] took 20 [[second]]s to half in [[Count Rate|count rate]]: |
| − | :'''Half life''' = 20 [[ | + | :'''Half life''' = 20 [[second]]s. |
| − | 2. Predict the [[Count Rate|count rate]] at 90 | + | 2. Predict the [[Count Rate|count rate]] at 90 [[second]]s. |
| − | :Since the '''half life''' is 20 | + | :Since the '''half life''' is 20 [[second]]s the [[Count Rate|count rate]] 20 [[second]]s earlier can be halved. :At 70 [[second]]s the [[Count Rate|count rate]] was 88Bq. Therefore at 90 [[second]]s the [[Count Rate|count rate]] would be: |
:88/2 = 44Bq | :88/2 = 44Bq | ||
| − | 3. Predict what the [[Count Rate|count rate]] was 10 | + | 3. Predict what the [[Count Rate|count rate]] was 10 [[second]]s before the [[sample]] was first tested. |
| − | :Since the '''half life''' is 20 | + | :Since the '''half life''' is 20 [[second]]s the [[Count Rate|count rate]] 20 [[second]]s later can be doubled. :At 10 [[second]]s the [[Count Rate|count rate]] was 707Bq. Therefore at -10 [[second]]s the [[Count Rate|count rate]] would have been: |
:707x2= 1414Bq | :707x2= 1414Bq | ||
| + | |||
| + | {| border="1" style="border-collapse:collapse" | ||
| + | | style="height:20px; width:100px; text-align:center;" |Time /min | ||
| + | | style="height:20px; width:50px; text-align:center;" |0 | ||
| + | | style="height:20px; width:50px; text-align:center;" |1 | ||
| + | | style="height:20px; width:50px; text-align:center;" |2 | ||
| + | | style="height:20px; width:50px; text-align:center;" |3 | ||
| + | | style="height:20px; width:50px; text-align:center;" |4 | ||
| + | | style="height:20px; width:50px; text-align:center;" |5 | ||
| + | | style="height:20px; width:50px; text-align:center;" |6 | ||
| + | | style="height:20px; width:50px; text-align:center;" |7 | ||
| + | | style="height:20px; width:50px; text-align:center;" |8 | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |Count rate /Bq | ||
| + | | style="height:20px; width:50px; text-align:center;" |5000 | ||
| + | | style="height:20px; width:50px; text-align:center;" |3968 | ||
| + | | style="height:20px; width:50px; text-align:center;" |3150 | ||
| + | | style="height:20px; width:50px; text-align:center;" |2500 | ||
| + | | style="height:20px; width:50px; text-align:center;" |1984 | ||
| + | | style="height:20px; width:50px; text-align:center;" |1575 | ||
| + | | style="height:20px; width:50px; text-align:center;" |1250 | ||
| + | | style="height:20px; width:50px; text-align:center;" |992 | ||
| + | | style="height:20px; width:50px; text-align:center;" |788 | ||
| + | |} | ||
| + | |||
| + | 1. Find the '''half life''' of this [[sample]] of [[radioactive]] [[isotope]]. | ||
| + | :The '''half life''' of of this [[sample]] of [[radioactive]] [[isotope]] can be found by halving the initial count rate. | ||
| + | |||
| + | :5000/2 = 2500Bq | ||
| + | |||
| + | :The [[isotope]] took 3 [[minute]]s to half in [[Count Rate|count rate]]: | ||
| + | :'''Half life''' = 3 [[minute]]s. | ||
| + | |||
| + | 2. Predict the [[Count Rate|count rate]] at 9 [[minute]]s. | ||
| + | :Since the '''half life''' is 3 [[minute]]s the [[Count Rate|count rate]] 3 [[minute]]s earlier can be halved. | ||
| + | :At 6 [[minute]]s the [[Count Rate|count rate]] was 1250Bq. Therefore at 9 [[minute]]s the [[Count Rate|count rate]] would be: | ||
| + | |||
| + | :1250/2 = 625Bq | ||
| + | |||
| + | 3. Predict what the [[Count Rate|count rate]] was 2 [[minute]]s before the [[sample]] was first tested. | ||
| + | :Since the '''half life''' is 3 [[minute]]s the [[Count Rate|count rate]] 3 [[minute]]s later can be doubled. | ||
| + | :At 1 minute the [[Count Rate|count rate]] was 3968Bq. Therefore at -2 [[minute]]s the [[Count Rate|count rate]] would have been: | ||
| + | |||
| + | :3968x2= 7936Bq | ||
| + | |||
| + | ==Key Stage 4 Higher== | ||
| + | ===Meaning=== | ||
| + | [[File:HalfLifeGraph1.png|right|300px|thumb|A [[graph]] showing the fraction of [[Unstable Isotope|unstable isotope]] left compared to the '''half life'''.]] | ||
| + | A '''half life''' is the time it takes for half of the [[Unstable Isotope|unstable isotopes]] in a [[radioactive]] sample to [[Radioactive Decay|decay]]. | ||
| + | |||
| + | ===About Half Life=== | ||
| + | : Every [[isotope]] has a unique '''half life'''. | ||
| + | : The '''half life''' of an [[isotope]] depends on how [[Stable Isotope|stable]] the [[isotope]] is. | ||
| + | : The '''half life''' of an [[isotope]] can be determined by measuring the [[emission]] of [[Ionising Radiation|ionising radiation]]. The time it takes for the [[Count Rate|count rate]] of [[Ionising Radiation|ionising radiation]] to halve, is the same as its '''half life'''. | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:UnstableIsotopeDecay.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:left;" |This [[diagram]] shows the number of [[Fermium]]-252 [[atom]]s halves each day, so the '''half life''' is 1 day. | ||
| + | |} | ||
| + | |||
| + | : The '''half life''' of an [[isotope]] can be found from plotting a [[graph]] of the amount or [[isotope]] or the [[Count Rate|count rate]] against the [[time]]. The '''half life''' is the time these take to halve from their initial value. | ||
| + | : The [[Count Rate|count rate]] of a sample of [[Unstable Isotope|unstable isotope]] can be predicted given its initial [[Count Rate|count rate]], its '''half life''' and the [[time]] it has been given. | ||
| + | |||
| + | ===Finding Half Life from a Graph=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |Find the '''half life''' from this [[graph]]. | ||
| + | | style="height:20px; width:300px; text-align:center;" |Find the '''half life''' from this [[graph]]. | ||
| + | |- | ||
| + | |[[File:HalfLifeGraph2.png|center|300px]] | ||
| + | |[[File:HalfLifeGraph3.png|center|300px]] | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |There is 100% of the [[isotope]] at the beginning. Draw a horizontal line from 50% to the curve. Draw a vertical line down from the curve to indicate the '''half life'''. | ||
| + | | style="height:20px; width:300px; text-align:center;" |The [[Count Rate|count rate]] began at 70,000Bq. Draw a horizontal line from 35,000 (half the initial count rate) to the curve. Draw a vertical line down from the curve to indicate the '''half life'''. | ||
| + | |- | ||
| + | |[[File:HalfLifeGraph2b.png|center|300px]] | ||
| + | |[[File:HalfLifeGraph3b.png|center|300px]] | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" | | ||
| + | '''Half Life''' = 4 [[minute]]s | ||
| + | | style="height:20px; width:300px; text-align:center;" | | ||
| + | '''Half Life''' = 6 [[hour]]s | ||
| + | |} | ||
| + | |||
| + | ===Equation=== | ||
| + | |||
| + | New Count Rate = (Initial Count Rate)/(2<sup>number of '''half lives'''</sup>) | ||
| + | |||
| + | <math>R = \frac{R_I}{2^n}</math> | ||
| + | |||
| + | Where | ||
| + | |||
| + | <math>R</math> = New [[Count Rate]] or number of [[atom]]s of an [[Unstable Isotope|unstable isotope]] left. | ||
| + | |||
| + | <math>R_I</math> = Initial [[Count Rate]] or number of [[atom]]s of an [[Unstable Isotope|unstable isotope]]. | ||
| + | |||
| + | <math>n</math> = The number of '''half lives'''. | ||
| + | |||
| + | ===Calculating Count Rate=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |An [[isotope]] has an initial [[Count Rate|count rate]] of 16000Bq and a '''half life''' of 2.5 days. Calculate the [[Count Rate|count rate]] of the [[isotope]] after 17.5 days. | ||
| + | | style="height:20px; width:300px; text-align:center;" |A [[radioactive]] [[sample]] begins with 576 [[atom]]s of an [[Unstable Isotope|unstable isotope]]. Given its '''half life''' is 300 seconds, calculate the number of [[atom]]s remaining after 25 minutes. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''1. Calculate the number of half lives which have occurred.''' | ||
| + | |||
| + | <math>17.5 ÷ 2.5 = 7</math> | ||
| + | |||
| + | n = 7 '''half lives''' | ||
| + | |||
| + | | style="height:20px; width:300px; text-align:center;" |'''1. Identify how many times the number of [[Unstable Isotope|unstable]] [[atom]]s has halved.''' | ||
| + | |||
| + | <math>25 \times 60 ÷ 300 = 5</math> | ||
| + | |||
| + | n = 5 '''half lives''' | ||
| + | |||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''2. Substitute the numbers into the equation and solve.''' | ||
| + | |||
| + | <math>R = \frac{R_I}{2^n}</math> | ||
| + | |||
| + | <math>R = \frac{16000}{2^7}</math> | ||
| + | |||
| + | <math>R = \frac{16000}{128}</math> | ||
| + | |||
| + | <math>R = 125Bq</math> | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''2. Substitute the numbers into the equation and solve.''' | ||
| + | |||
| + | <math>R = \frac{R_I}{2^n}</math> | ||
| + | |||
| + | <math>R = \frac{576}{2^5}</math> | ||
| + | |||
| + | <math>R = \frac{576}{32}</math> | ||
| + | |||
| + | <math>R = 18atoms</math> | ||
| + | |} | ||
| + | |||
| + | ===Calculating Half Life=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |An [[isotope]] had a [[Count Rate|count rate]] of 200Bq and 90 days later has a [[Count Rate|count rate]] of 25. Calculate the '''half life''' of this [[isotope]]. | ||
| + | | style="height:20px; width:300px; text-align:center;" |A [[radioactive]] [[sample]] begins with 224 [[atom]]s of an [[Unstable Isotope|unstable isotope]] 18 [[minute]]s later there are only 14 [[atom]]s of the [[Unstable Isotope|unstable isotope]] left. Calculate the '''half life''' of this [[isotope]]. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''1. Identify how many times the [[Count Rate|count rate]] has halved.''' | ||
| + | |||
| + | <math>200 ÷ 2 = 100</math> | ||
| + | |||
| + | <math>100 ÷ 2 = 50</math> | ||
| + | |||
| + | <math>50 ÷ 2 = 25</math> | ||
| + | |||
| + | The [[Count Rate|count rate]] has halved 3 times. | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''1. Identify how many times the number of [[Unstable Isotope|unstable]] [[atom]]s has halved.''' | ||
| + | |||
| + | <math>224 ÷ 2 = 112</math> | ||
| + | |||
| + | <math>112 ÷ 2 = 56</math> | ||
| + | |||
| + | <math>56 ÷ 2 = 28</math> | ||
| + | |||
| + | <math>56 ÷ 2 = 28</math> | ||
| + | |||
| + | The number of [[Unstable Isotope|unstable]] [[atom]]s has halved 4 times. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''2. Divide the time taken by the number of times the [[Count Rate|count rate]] has halved.''' | ||
| + | |||
| + | <math>90 ÷ 3 = 30</math> | ||
| + | |||
| + | '''Half Life''' = 30 days. | ||
| + | | style="height:20px; width:300px; text-align:center;" |'''2. Divide the time taken by the number of times the number of [[Unstable Isotope|unstable]] [[atom]]s has halved.''' | ||
| + | |||
| + | <math>18 ÷ 4 = 4.5</math> | ||
| + | |||
| + | '''Half Life''' = 4.5 [[minute]]s | ||
| + | |} | ||
| + | |||
| + | ===Finding Half Life and Count Rate from a Table=== | ||
| + | {| border="1" style="border-collapse:collapse" | ||
| + | | style="height:20px; width:100px; text-align:center;" |Time /s | ||
| + | | style="height:20px; width:50px; text-align:center;" |0 | ||
| + | | style="height:20px; width:50px; text-align:center;" |10 | ||
| + | | style="height:20px; width:50px; text-align:center;" |20 | ||
| + | | style="height:20px; width:50px; text-align:center;" |30 | ||
| + | | style="height:20px; width:50px; text-align:center;" |40 | ||
| + | | style="height:20px; width:50px; text-align:center;" |50 | ||
| + | | style="height:20px; width:50px; text-align:center;" |60 | ||
| + | | style="height:20px; width:50px; text-align:center;" |70 | ||
| + | | style="height:20px; width:50px; text-align:center;" |80 | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |Count rate /Bq | ||
| + | | style="height:20px; width:50px; text-align:center;" |1000 | ||
| + | | style="height:20px; width:50px; text-align:center;" |707 | ||
| + | | style="height:20px; width:50px; text-align:center;" |500 | ||
| + | | style="height:20px; width:50px; text-align:center;" |353 | ||
| + | | style="height:20px; width:50px; text-align:center;" |250 | ||
| + | | style="height:20px; width:50px; text-align:center;" |176 | ||
| + | | style="height:20px; width:50px; text-align:center;" |125 | ||
| + | | style="height:20px; width:50px; text-align:center;" |88 | ||
| + | | style="height:20px; width:50px; text-align:center;" |62 | ||
| + | |} | ||
| + | |||
| + | 1. Find the '''half life''' of this [[sample]] of [[radioactive]] [[isotope]]. | ||
| + | :The '''half life''' of of this [[sample]] of [[radioactive]] [[isotope]] can be found by halving the initial count rate. | ||
| + | |||
| + | :1000/2 = 500Bq | ||
| + | |||
| + | :The [[isotope]] took 20 [[second]]s to half in [[Count Rate|count rate]]: | ||
| + | :'''Half life''' = 20 [[second]]s. | ||
| + | |||
| + | 2. Predict the [[Count Rate|count rate]] at 90 [[second]]s. | ||
| + | :Since the '''half life''' is 20 [[second]]s the [[Count Rate|count rate]] 20 [[second]]s earlier can be halved. :At 70 [[second]]s the [[Count Rate|count rate]] was 88Bq. Therefore at 90 [[second]]s the [[Count Rate|count rate]] would be: | ||
| + | |||
| + | :88/2 = 44Bq | ||
| + | |||
| + | 3. Predict what the [[Count Rate|count rate]] was 10 [[second]]s before the [[sample]] was first tested. | ||
| + | :Since the '''half life''' is 20 [[second]]s the [[Count Rate|count rate]] 20 [[second]]s later can be doubled. :At 10 [[second]]s the [[Count Rate|count rate]] was 707Bq. Therefore at -10 [[second]]s the [[Count Rate|count rate]] would have been: | ||
| + | |||
| + | :707x2= 1414Bq | ||
| + | |||
| + | {| border="1" style="border-collapse:collapse" | ||
| style="height:20px; width:100px; text-align:center;" |Time /min | | style="height:20px; width:100px; text-align:center;" |Time /min | ||
| style="height:20px; width:50px; text-align:center;" |0 | | style="height:20px; width:50px; text-align:center;" |0 | ||
| Line 85: | Line 365: | ||
| style="height:20px; width:50px; text-align:center;" |788 | | style="height:20px; width:50px; text-align:center;" |788 | ||
|} | |} | ||
| + | |||
| + | 1. Find the '''half life''' of this [[sample]] of [[radioactive]] [[isotope]]. | ||
| + | :The '''half life''' of of this [[sample]] of [[radioactive]] [[isotope]] can be found by halving the initial count rate. | ||
| + | |||
| + | :5000/2 = 2500Bq | ||
| + | |||
| + | :The [[isotope]] took 3 [[minute]]s to half in [[Count Rate|count rate]]: | ||
| + | :'''Half life''' = 3 [[minute]]s. | ||
| + | |||
| + | 2. Predict the [[Count Rate|count rate]] at 9 [[minute]]s. | ||
| + | :Since the '''half life''' is 3 [[minute]]s the [[Count Rate|count rate]] 3 [[minute]]s earlier can be halved. | ||
| + | :At 6 [[minute]]s the [[Count Rate|count rate]] was 1250Bq. Therefore at 9 [[minute]]s the [[Count Rate|count rate]] would be: | ||
| + | |||
| + | :1250/2 = 625Bq | ||
| + | |||
| + | 3. Predict what the [[Count Rate|count rate]] was 2 [[minute]]s before the [[sample]] was first tested. | ||
| + | :Since the '''half life''' is 3 [[minute]]s the [[Count Rate|count rate]] 3 [[minute]]s later can be doubled. | ||
| + | :At 1 minute the [[Count Rate|count rate]] was 3968Bq. Therefore at -2 [[minute]]s the [[Count Rate|count rate]] would have been: | ||
| + | |||
| + | :3968x2= 7936Bq | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09977 ''Half-life, page 200, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac236 ''Half-life, page 46, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Half-life, pages 100-101, 109, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Half-life, pages 118-120, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Half-life, pages 118-19, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Half-life, pages 130-132, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Half-life; definition, page 118, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Half-lives, page 99, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Half-lives, pages 248-9, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Half-lives; and stability, page 100, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Half-lives; measurement of, pages 99-100, GCSE Physics, Hodder, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Half-life, page 176, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945733/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945733&linkCode=as2&tag=nrjc-21&linkId=2a2dbec9db6bf5766c0458d908fa0a52 ''Half-life, page 53, GCSE Physics; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Half-life, pages 161-163, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Half-life; effect on safety, page 167, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Half-lives, pages 102-103, GCSE Physics, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Half-lives, pages 366-367, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359837/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359837&linkCode=as2&tag=nrjc-21&linkId=3c4229e8b023b2b60768e7ea2307cc6f ''Half-life, pages 176-177, 183, Gateway GCSE Physics, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Half-lives, page 199, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945687/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945687&linkCode=as2&tag=nrjc-21&linkId=9a598e52189317a20311d7a632747bc9 ''Half-lives, pages 77, 79, Gateway GCSE Physics; The Revision Guide, CGP, OCR ''] | ||
Latest revision as of 16:58, 11 December 2019
Key Stage 4 Foundation
Meaning
A half life is the time it takes for half of the unstable isotopes in a radioactive sample to decay.
About Half Life
- Every isotope has a unique half life.
- The half life of an isotope depends on how stable the isotope is.
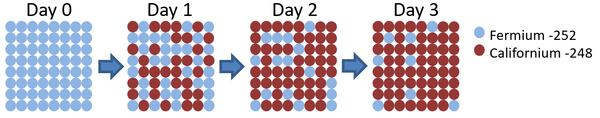
- The half life of an isotope can be determined by measuring the emission of ionising radiation. The time it takes for the count rate of ionising radiation to halve, is the same as its half life.
| This diagram shows the number of Fermium-252 atoms halves each day, so the half life is 1 day. |
- The half life of an isotope can be found from plotting a graph of the amount or isotope or the count rate against the time. The half life is the time these take to halve from their initial value.
- The count rate of a sample of unstable isotope can be predicted given its initial count rate, its half life and the time it has been given.
Finding Half Life from a Graph
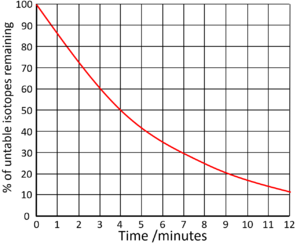
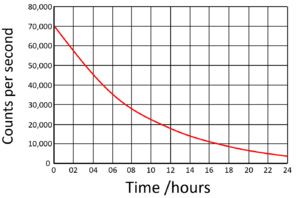
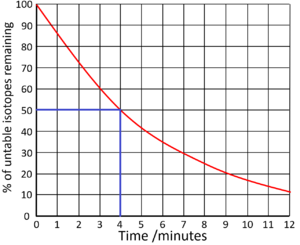
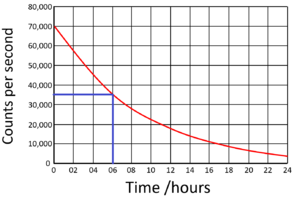
| Find the half life from this graph. | Find the half life from this graph. |
| There is 100% of the isotope at the beginning. Draw a horizontal line from 50% to the curve. Draw a vertical line down from the curve to indicate the half life. | The count rate began at 70,000Bq. Draw a horizontal line from 35,000 (half the initial count rate) to the curve. Draw a vertical line down from the curve to indicate the half life. |
|
Half Life = 4 minutes |
Half Life = 6 hours |
Calculating Half Life
| An isotope had a count rate of 200Bq and 90 days later has a count rate of 25. Calculate the half life of this isotope. | A radioactive sample begins with 224 atoms of an unstable isotope 18 minutes later there are only 14 atoms of the unstable isotope left. Calculate the half life of this isotope. |
| 1. Identify how many times the count rate has halved.
\(200 ÷ 2 = 100\) \(100 ÷ 2 = 50\) \(50 ÷ 2 = 25\) The count rate has halved 3 times. |
1. Identify how many times the number of unstable atoms has halved.
\(224 ÷ 2 = 112\) \(112 ÷ 2 = 56\) \(56 ÷ 2 = 28\) \(56 ÷ 2 = 28\) |
| 2. Divide the time taken by the number of times the count rate has halved.
\(90 ÷ 3 = 30\) Half Life = 30 days. |
2. Divide the time taken by the number of times the number of unstable atoms has halved.
\(18 ÷ 4 = 4.5\) Half Life = 4.5 minutes |
Finding Half Life and Count Rate from a Table
| Time /s | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Count rate /Bq | 1000 | 707 | 500 | 353 | 250 | 176 | 125 | 88 | 62 |
1. Find the half life of this sample of radioactive isotope.
- The half life of of this sample of radioactive isotope can be found by halving the initial count rate.
- 1000/2 = 500Bq
- The isotope took 20 seconds to half in count rate:
- Half life = 20 seconds.
2. Predict the count rate at 90 seconds.
- Since the half life is 20 seconds the count rate 20 seconds earlier can be halved. :At 70 seconds the count rate was 88Bq. Therefore at 90 seconds the count rate would be:
- 88/2 = 44Bq
3. Predict what the count rate was 10 seconds before the sample was first tested.
- Since the half life is 20 seconds the count rate 20 seconds later can be doubled. :At 10 seconds the count rate was 707Bq. Therefore at -10 seconds the count rate would have been:
- 707x2= 1414Bq
| Time /min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Count rate /Bq | 5000 | 3968 | 3150 | 2500 | 1984 | 1575 | 1250 | 992 | 788 |
1. Find the half life of this sample of radioactive isotope.
- The half life of of this sample of radioactive isotope can be found by halving the initial count rate.
- 5000/2 = 2500Bq
- The isotope took 3 minutes to half in count rate:
- Half life = 3 minutes.
2. Predict the count rate at 9 minutes.
- Since the half life is 3 minutes the count rate 3 minutes earlier can be halved.
- At 6 minutes the count rate was 1250Bq. Therefore at 9 minutes the count rate would be:
- 1250/2 = 625Bq
3. Predict what the count rate was 2 minutes before the sample was first tested.
- Since the half life is 3 minutes the count rate 3 minutes later can be doubled.
- At 1 minute the count rate was 3968Bq. Therefore at -2 minutes the count rate would have been:
- 3968x2= 7936Bq
Key Stage 4 Higher
Meaning
A half life is the time it takes for half of the unstable isotopes in a radioactive sample to decay.
About Half Life
- Every isotope has a unique half life.
- The half life of an isotope depends on how stable the isotope is.
- The half life of an isotope can be determined by measuring the emission of ionising radiation. The time it takes for the count rate of ionising radiation to halve, is the same as its half life.
| This diagram shows the number of Fermium-252 atoms halves each day, so the half life is 1 day. |
- The half life of an isotope can be found from plotting a graph of the amount or isotope or the count rate against the time. The half life is the time these take to halve from their initial value.
- The count rate of a sample of unstable isotope can be predicted given its initial count rate, its half life and the time it has been given.
Finding Half Life from a Graph
| Find the half life from this graph. | Find the half life from this graph. |
| There is 100% of the isotope at the beginning. Draw a horizontal line from 50% to the curve. Draw a vertical line down from the curve to indicate the half life. | The count rate began at 70,000Bq. Draw a horizontal line from 35,000 (half the initial count rate) to the curve. Draw a vertical line down from the curve to indicate the half life. |
|
Half Life = 4 minutes |
Half Life = 6 hours |
Equation
New Count Rate = (Initial Count Rate)/(2number of half lives)
\(R = \frac{R_I}{2^n}\)
Where
\(R\) = New Count Rate or number of atoms of an unstable isotope left.
\(R_I\) = Initial Count Rate or number of atoms of an unstable isotope.
\(n\) = The number of half lives.
Calculating Count Rate
| An isotope has an initial count rate of 16000Bq and a half life of 2.5 days. Calculate the count rate of the isotope after 17.5 days. | A radioactive sample begins with 576 atoms of an unstable isotope. Given its half life is 300 seconds, calculate the number of atoms remaining after 25 minutes. |
| 1. Calculate the number of half lives which have occurred.
\(17.5 ÷ 2.5 = 7\) n = 7 half lives |
1. Identify how many times the number of unstable atoms has halved.
\(25 \times 60 ÷ 300 = 5\) n = 5 half lives |
| 2. Substitute the numbers into the equation and solve.
\(R = \frac{R_I}{2^n}\) \(R = \frac{16000}{2^7}\) \(R = \frac{16000}{128}\) \(R = 125Bq\) |
2. Substitute the numbers into the equation and solve.
\(R = \frac{R_I}{2^n}\) \(R = \frac{576}{2^5}\) \(R = \frac{576}{32}\) \(R = 18atoms\) |
Calculating Half Life
| An isotope had a count rate of 200Bq and 90 days later has a count rate of 25. Calculate the half life of this isotope. | A radioactive sample begins with 224 atoms of an unstable isotope 18 minutes later there are only 14 atoms of the unstable isotope left. Calculate the half life of this isotope. |
| 1. Identify how many times the count rate has halved.
\(200 ÷ 2 = 100\) \(100 ÷ 2 = 50\) \(50 ÷ 2 = 25\) The count rate has halved 3 times. |
1. Identify how many times the number of unstable atoms has halved.
\(224 ÷ 2 = 112\) \(112 ÷ 2 = 56\) \(56 ÷ 2 = 28\) \(56 ÷ 2 = 28\) |
| 2. Divide the time taken by the number of times the count rate has halved.
\(90 ÷ 3 = 30\) Half Life = 30 days. |
2. Divide the time taken by the number of times the number of unstable atoms has halved.
\(18 ÷ 4 = 4.5\) Half Life = 4.5 minutes |
Finding Half Life and Count Rate from a Table
| Time /s | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Count rate /Bq | 1000 | 707 | 500 | 353 | 250 | 176 | 125 | 88 | 62 |
1. Find the half life of this sample of radioactive isotope.
- The half life of of this sample of radioactive isotope can be found by halving the initial count rate.
- 1000/2 = 500Bq
- The isotope took 20 seconds to half in count rate:
- Half life = 20 seconds.
2. Predict the count rate at 90 seconds.
- Since the half life is 20 seconds the count rate 20 seconds earlier can be halved. :At 70 seconds the count rate was 88Bq. Therefore at 90 seconds the count rate would be:
- 88/2 = 44Bq
3. Predict what the count rate was 10 seconds before the sample was first tested.
- Since the half life is 20 seconds the count rate 20 seconds later can be doubled. :At 10 seconds the count rate was 707Bq. Therefore at -10 seconds the count rate would have been:
- 707x2= 1414Bq
| Time /min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Count rate /Bq | 5000 | 3968 | 3150 | 2500 | 1984 | 1575 | 1250 | 992 | 788 |
1. Find the half life of this sample of radioactive isotope.
- The half life of of this sample of radioactive isotope can be found by halving the initial count rate.
- 5000/2 = 2500Bq
- The isotope took 3 minutes to half in count rate:
- Half life = 3 minutes.
2. Predict the count rate at 9 minutes.
- Since the half life is 3 minutes the count rate 3 minutes earlier can be halved.
- At 6 minutes the count rate was 1250Bq. Therefore at 9 minutes the count rate would be:
- 1250/2 = 625Bq
3. Predict what the count rate was 2 minutes before the sample was first tested.
- Since the half life is 3 minutes the count rate 3 minutes later can be doubled.
- At 1 minute the count rate was 3968Bq. Therefore at -2 minutes the count rate would have been:
- 3968x2= 7936Bq
References
AQA
- Half-life, page 200, GCSE Combined Science; The Revision Guide, CGP, AQA
- Half-life, page 46, GCSE Physics; The Revision Guide, CGP, AQA
- Half-life, pages 100-101, 109, GCSE Physics; Third Edition, Oxford University Press, AQA
- Half-life, pages 118-120, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Half-life, pages 118-19, GCSE Physics; Student Book, Collins, AQA
- Half-life, pages 130-132, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Half-life; definition, page 118, GCSE Physics; Student Book, Collins, AQA
- Half-lives, page 99, GCSE Physics, Hodder, AQA
- Half-lives, pages 248-9, GCSE Combined Science Trilogy 1, Hodder, AQA
- Half-lives; and stability, page 100, GCSE Physics, Hodder, AQA
- Half-lives; measurement of, pages 99-100, GCSE Physics, Hodder, AQA
Edexcel
- Half-life, page 176, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Half-life, page 53, GCSE Physics; The Revision Guide, CGP, Edexcel
- Half-life, pages 161-163, GCSE Physics, CGP, Edexcel
- Half-life; effect on safety, page 167, GCSE Physics, CGP, Edexcel
- Half-lives, pages 102-103, GCSE Physics, Pearson Edexcel
- Half-lives, pages 366-367, GCSE Combined Science, Pearson Edexcel