Difference between revisions of "Litmus Paper"
(Created page with "==Key Stage 3== ===Meaning=== right|300px|thumb|Two pieces of '''litmus paper''' dipped in an [[acid showing the red '''litmus''' stayed red but the b...") |
|||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
===About Litmus Paper=== | ===About Litmus Paper=== | ||
: '''Litmus paper''' comes in two colours: Red and Blue. | : '''Litmus paper''' comes in two colours: Red and Blue. | ||
| − | : '''Litmus | + | : '''Litmus paper''' is a very simple [[Indicator (Chemistry)|indicator]] as it can only tell if something is [[acid]] or [[alkali]] but it cannot tell the exact [[pH]] of a [[solution]]. |
| − | : ''' | + | : '''Litmus paper''' cannot be used on a [[base]] unless it is in [[solution]]. |
| − | + | ||
| − | + | {| class="wikitable" | |
| − | + | |- | |
| − | + | |[[File:RedLitmusAcid.png|center|200px]] | |
| − | + | |[[File:RedLitmusAlkali.png|center|200px]] | |
| − | + | |[[File:RedLitmusNeutral.png|center|200px]] | |
| + | |- | ||
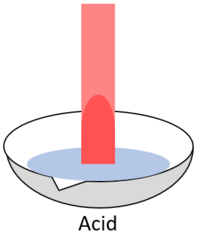
| + | | style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in an [[acid]] it stays red. | ||
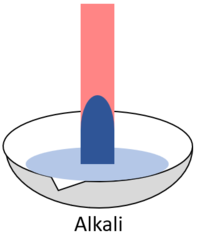
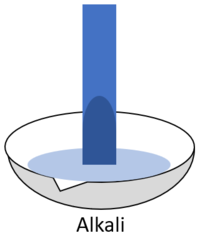
| + | | style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in an [[alkali]] it turns blue. | ||
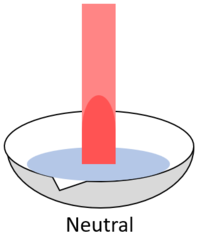
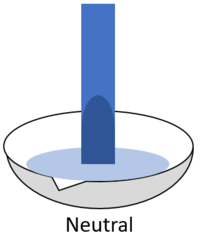
| + | | style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in a [[Neutral (Chemistry)|neutral]] [[solution]] it stays red. | ||
| + | |- | ||
| + | |[[File:BlueLitmusAcid.png|center|200px]] | ||
| + | |[[File:BlueLitmusAlkali.png|center|200px]] | ||
| + | |[[File:BlueLitmusNeutral.png|center|200px]] | ||
| + | |- | ||
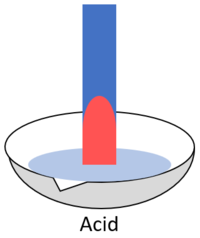
| + | | style="height:20px; width:200px; text-align:center;" |When blue '''litmus''' is placed in an [[acid]] it turns red. | ||
| + | | style="height:20px; width:200px; text-align:center;" |When blue '''litmus''' is placed in an [[alkali]] it stays blue. | ||
| + | | style="height:20px; width:200px; text-align:center;" |When blue '''litmus''' is placed in a [[Neutral (Chemistry)|neutral]] [[solution]] it stays blue. | ||
| + | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Litmus paper, pages 74, 95, 110, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Litmus, page 43, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Litmus, pages 119, 186, 213, 275, 323, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Litmus paper, pages 146, 267, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Litmus paper, pages 58, 103, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
Latest revision as of 08:58, 14 December 2019
Key Stage 3
Meaning
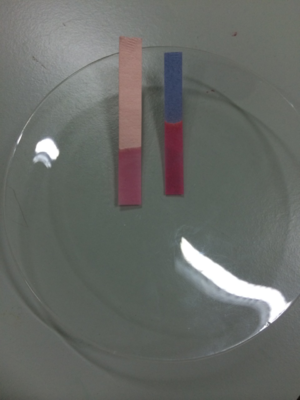
Two pieces of litmus paper dipped in an acid showing the red litmus stayed red but the blue litmus turned red.
Litmus paper is a piece of paper coloured with a dye that turns red in acid and blue in alkali.
About Litmus Paper
- Litmus paper comes in two colours: Red and Blue.
- Litmus paper is a very simple indicator as it can only tell if something is acid or alkali but it cannot tell the exact pH of a solution.
- Litmus paper cannot be used on a base unless it is in solution.
| When red litmus is placed in an acid it stays red. | When red litmus is placed in an alkali it turns blue. | When red litmus is placed in a neutral solution it stays red. |
| When blue litmus is placed in an acid it turns red. | When blue litmus is placed in an alkali it stays blue. | When blue litmus is placed in a neutral solution it stays blue. |
References
Edexcel
- Litmus paper, pages 74, 95, 110, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Litmus, page 43, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Litmus, pages 119, 186, 213, 275, 323, GCSE Chemistry, CGP, Edexcel