Difference between revisions of "Methanol"
| (4 intermediate revisions by 2 users not shown) | |||
| Line 26: | Line 26: | ||
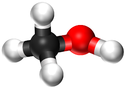
|[[File:BallandStickMethanol.png|center|125px]] | |[[File:BallandStickMethanol.png|center|125px]] | ||
|} | |} | ||
| − | |||
: The [[combustion]] of [[methanol]] [[product|produces]] [[Carbon Dioxide]] and [[Water]]. | : The [[combustion]] of [[methanol]] [[product|produces]] [[Carbon Dioxide]] and [[Water]]. | ||
: [[Methanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | : [[Methanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| − | + | ||
| − | : [[ | + | <math>2CH_3OH + 3O_2 → 2CO_2 + 4H_2O</math> |
| − | + | ||
| + | : [[Methanol]] can also be [[oxidise]]d at low [[temperature]]s to [[product|produce]] [[Water]] and the [[Carboxylic Acid]]; [[Methanoic Acid]]. | ||
| + | |||
| + | <math>CH_3OH + O_2 → CHOOH + H_2O</math> | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Methanol, page 238, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Methanol, page 242, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Methanol, pages 160, 162, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Methanol, pages 190-191, 234-235, 243-244, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 11:15, 14 December 2019
Contents
Key Stage 3
Meaning
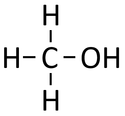
Methanol is a compound with chemical formula CH3OH.
About Methanol
- Methanol is a transparent liquid (at room temperature).
- Methanol can be oxidised to produce Carbon Dioxide and Water.
- Methanol + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Methanol is an alcohol molecule with chemical formula CH3OH.
About Methanol
- Methanol is a transparent liquid (at STP).
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| CH3OH | CH3OH |
- The combustion of methanol produces Carbon Dioxide and Water.
- Methanol + Oxygen → Carbon Dioxide + Water
\(2CH_3OH + 3O_2 → 2CO_2 + 4H_2O\)
- Methanol can also be oxidised at low temperatures to produce Water and the Carboxylic Acid; Methanoic Acid.
\(CH_3OH + O_2 → CHOOH + H_2O\)
References
AQA
- Methanol, page 238, GCSE Chemistry, CGP, AQA
- Methanol, page 242, GCSE Chemistry; Student Book, Collins, AQA
- Methanol, pages 160, 162, GCSE Chemistry; Third Edition, Oxford University Press, AQA