Difference between revisions of "Thermal Conduction"
| Line 5: | Line 5: | ||
|- | |- | ||
|[[File:ThermalConduction.gif|center]] | |[[File:ThermalConduction.gif|center]] | ||
| + | |- | ||
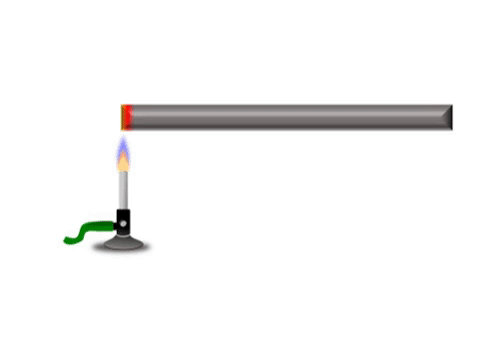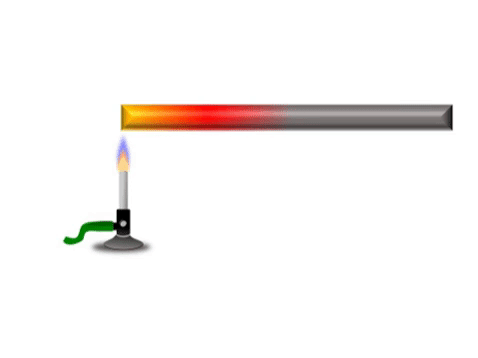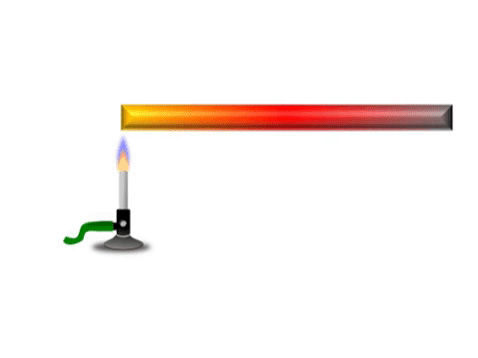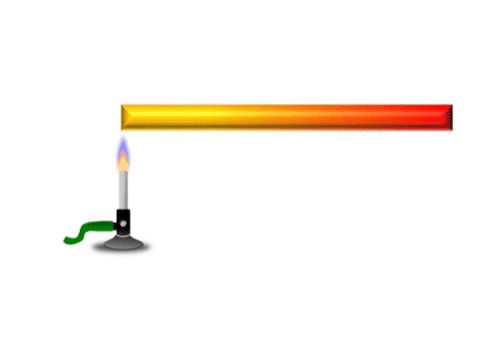
| + | | style="height:20px; width:200px; text-align:center;" |Grey represents the metal bar at a cold [[temperature]]. The yellow represents the highest [[temperature]] and red represents a [[temperature]] between yellow and grey. | ||
|} | |} | ||
| Line 10: | Line 12: | ||
: Of the three [[State of Matter|states of matter]], [[solid]]s are the best '''conductors''' of [[Thermal Energy Store|thermal energy]]. | : Of the three [[State of Matter|states of matter]], [[solid]]s are the best '''conductors''' of [[Thermal Energy Store|thermal energy]]. | ||
: [[Metal]]s are better '''[[Thermal Conductor|thermal conductors]]''' than [[Non-metal|non-metals]] because [[metal]]s have [[delocalised electron|free electrons]] that can pass along [[Thermal Energy|thermal energy]]. | : [[Metal]]s are better '''[[Thermal Conductor|thermal conductors]]''' than [[Non-metal|non-metals]] because [[metal]]s have [[delocalised electron|free electrons]] that can pass along [[Thermal Energy|thermal energy]]. | ||
| − | |||
Revision as of 15:32, 10 October 2018
Key Stage 3
Meaning
Conduction is when thermal energy passes along a material from the hotter region to the cooler region.
| Grey represents the metal bar at a cold temperature. The yellow represents the highest temperature and red represents a temperature between yellow and grey. |
About Thermal Conduction
- Of the three states of matter, solids are the best conductors of thermal energy.
- Metals are better thermal conductors than non-metals because metals have free electrons that can pass along thermal energy.