Difference between revisions of "Melting"
(→Key Stage 4) |
(→About Melting) |
||
| Line 69: | Line 69: | ||
|[[File:MeltingGraph.png|center|500px]] | |[[File:MeltingGraph.png|center|500px]] | ||
|- | |- | ||
| − | | style="height:20px; width:500px; text-align:left;" |As a [[solid]] is [[heated]] the [[Internal Energy|internal energy]] increases. As the [[solid]] '''melts''' the [[temperature]] of the [[substance]] stays the same. | + | | style="height:20px; width:500px; text-align:left;" |As a [[solid]] is [[heated]] the [[Internal Energy|internal energy]] increases. As the [[solid]] '''melts''' the [[temperature]] of the [[substance]] stays the same but the [[Potential Energy|potential energy]] of the [[particle]]s continues to increase. |
|} | |} | ||
Revision as of 11:24, 27 December 2018
Contents
Key Stage 2
Meaning
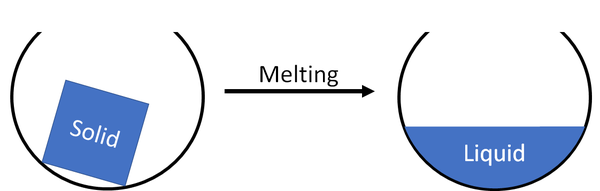
Melting is when a solid turns into a liquid.
- Verb: To melt
- Present Participle: Melting
| A solid will melt and become a liquid. |
About Melting
- Most solids can be melted to become a liquid.
- Melting is a reversible process. When a solid melts you can always freeze it back into a solid.
- You may have seen these solids melt
- Ice
- Wax
- Chocolate
- Butter
Examples
| Some ice cubes melt to make water. | Wax melts because of the flame. |
| Chocolate can melt in your mouth because your mouth is warm. | You can melt butter in a frying pan. |
Key Stage 3
Meaning
Melting is an endothermic process in which a solid turns into a liquid.
About Melting
- Most solids can be melted to become a liquid.
- Melting is a reversible process. When a solid melts you can always freeze it back into a solid.
- A solid can be melted by heating it.
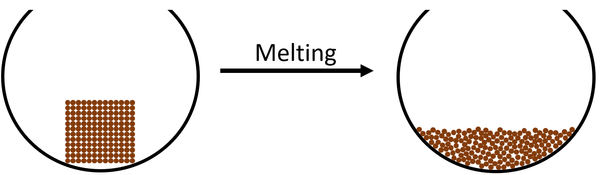
| The particles in the solid vibrate faster until they vibrate fast enough that they break the bonds holding them in fixed positions. The particles become able to move past each other but are still touching which makes the state a liquid. |
Key Stage 4
Meaning
Melting is an endothermic physical change in which a solid turns into a liquid.
About Melting
- Melting happens when the particles in a solid break bonds holding them in fixed positions as they gain potential energy.
- The temperature at which a substance melts is called its melting point.
- Melting is an endothermic process, which means it needs to absorb energy to take place.
- Melting is a physical change, which means it is reversible and does not produce new chemicals.
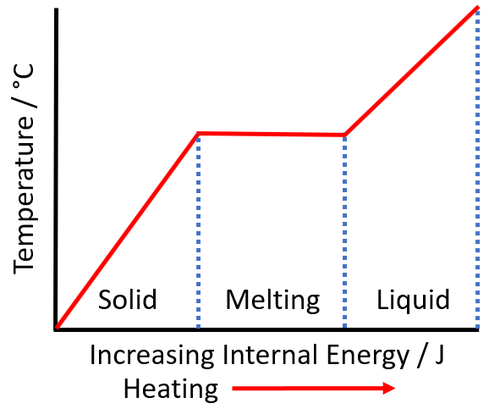
| As a solid is heated the internal energy increases. As the solid melts the temperature of the substance stays the same but the potential energy of the particles continues to increase. |