Difference between revisions of "Empirical Formula"
| Line 13: | Line 13: | ||
: '''Empirical formulae''' are calculated from the amount of [[atom]]s in a [[Chemical Reaction|chemical reaction]]. | : '''Empirical formulae''' are calculated from the amount of [[atom]]s in a [[Chemical Reaction|chemical reaction]]. | ||
: The number of [[atom]]s can be found if you know the [[mass]] of different [[element]]s and the [[Relative Atomic Mass|relative atomic mass]] of the [[element]]s in the [[Chemical Reaction|reaction]]. | : The number of [[atom]]s can be found if you know the [[mass]] of different [[element]]s and the [[Relative Atomic Mass|relative atomic mass]] of the [[element]]s in the [[Chemical Reaction|reaction]]. | ||
| + | |||
| + | ===Finding the Empirical Formula=== | ||
| + | {| class="wikitable" | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Number of Atoms''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Picture''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Structural Diagram''' | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |100 [[atom]]s of [[Hydrogen]] [[Chemical Reaction|react]] completely with 50 [[atom]]s of [[Oxygen]]. | ||
| + | |[[File:SkeletalFormulaButane.png|center|200px]] | ||
| + | |[[File:BallandStickButan12diol.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | The [[ratio]] of [[Hydrogen]] [[atom]]s to [[Oxygen]] [[atom]]s | ||
| + | |||
| + | H:O | ||
| + | |||
| + | 100:50 | ||
| + | |||
| + | 2:1 | ||
| + | |||
| + | So the '''empirical formula''' is H<sub>2</sub>O | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
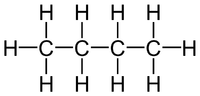
| + | In this [[diagram]] there are 4 [[Carbon]] [[atom]]s, 10 [[Hydrogen]] [[atom]]s. | ||
| + | |||
| + | The [[ratio]] of [[atom]]s is: | ||
| + | |||
| + | C:H | ||
| + | |||
| + | 4:10 | ||
| + | |||
| + | 2:5 | ||
| + | |||
| + | So the '''empirical formula''' is C<sub>2</sub>H<sub>5</sub> | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
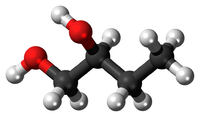
| + | In this [[diagram]] there are 4 [[Carbon]] [[atom]]s, 10 [[Hydrogen]] [[atom]]s and 2 [[Oxygen]] [[atom]]s. | ||
| + | |||
| + | The [[ratio]] of [[atom]]s is: | ||
| + | |||
| + | C:H:O | ||
| + | |||
| + | 4:10:2 | ||
| + | |||
| + | 2:5:1 | ||
| + | |||
| + | So the '''empirical formula''' is C<sub>2</sub>H<sub>5</sub>O | ||
| + | |||
| + | |||
| + | |} | ||
Revision as of 16:28, 2 January 2019
Key Stage 4
Meaning
An empirical formula is the simplest ratio of the different types of atom in a compound.
About Empirical Formulae
The empirical formula of a compound may not be the same as the chemical formula:
- Ethane - Chemical Formula C2H6, Empirical Formula CH3
- Ethene - Chemical Formula C2H4, Empirical Formula CH2
- Propene - Chemical Formula C3H6, Empirical Formula CH2
- Glucose - Chemical Formula C6H12O6, Empirical Formula CH2O
- Lactic Acid - Chemical Formula C3H6O3, Empirical Formula CH2O
- Empirical formulae are calculated from the amount of atoms in a chemical reaction.
- The number of atoms can be found if you know the mass of different elements and the relative atomic mass of the elements in the reaction.
Finding the Empirical Formula
| Number of Atoms | Picture | Structural Diagram |
| 100 atoms of Hydrogen react completely with 50 atoms of Oxygen. | ||
|
The ratio of Hydrogen atoms to Oxygen atoms H:O 100:50 2:1 So the empirical formula is H2O |
In this diagram there are 4 Carbon atoms, 10 Hydrogen atoms. C:H 4:10 2:5 So the empirical formula is C2H5 |
In this diagram there are 4 Carbon atoms, 10 Hydrogen atoms and 2 Oxygen atoms. C:H:O 4:10:2 2:5:1 So the empirical formula is C2H5O
|