Difference between revisions of "Bond Energy"
(Created page with "==Key Stage 4== ===Meaning=== '''Bond Energy''' is the energy needed to break a chemical bond between two atoms. ===About Bond Energy=== : When Ch...") |
(→Examples) |
||
| Line 50: | Line 50: | ||
| style="height:20px; width:150px; text-align:center;" |391 | | style="height:20px; width:150px; text-align:center;" |391 | ||
|} | |} | ||
| + | |||
| + | These can be used to calculate the [[energy]] released per [[mole]] in a [[Chemical Reaction|chemical reaction]]. | ||
| + | |||
| + | '''Example 1''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
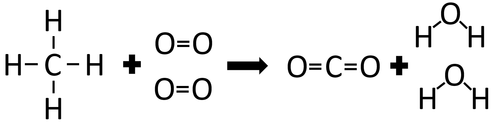
| + | |[[File:StructuralDiagramMethane+Oxygen.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |In the [[Chemical Reaction|reaction]] between [[Methane]] and [[Oxygen]] the [[Chemical Bond|chemical bonds]] in the [[reactant]]s must be broken first before the [[Chemical Bond|bonds]] in the [[product]]s are formed. | ||
| + | |} | ||
| + | |||
| + | There are 4 C-H [[Chemical Bond|bonds]] and 2 O=O [[Chemical Bond|bonds]]. | ||
| + | |||
| + | 4 x 413 + 2 x 498 = 2648kJ | ||
| + | |||
| + | Therefore 2648kJ/mol are needed to break the [[Chemical Bond|bonds]] in the [[reactant]]s. | ||
| + | |||
| + | There are 2 C=O [[Chemical Bond|bonds]] and 4 O-H [[Chemical Bond|bonds]]. | ||
| + | |||
| + | 2 x 799 + 4 x 464 = 3454kJ | ||
| + | |||
| + | Therefore 3454kJ/mol is released when the [[Chemical Bond|bonds]] in the [[product]]s form. | ||
| + | |||
| + | Once the [[Chemical Reaction|reaction]] is complete 806kJ will be released. | ||
| + | |||
| + | '''Example 2''' | ||
| + | {| class="wikitable" | ||
| + | |- | ||
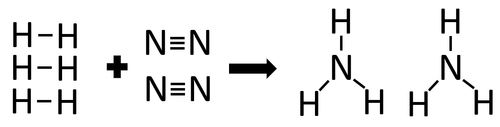
| + | |[[File:StructuralDiagramHydrogen+Nitrogen.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |In the [[Chemical Reaction|reaction]] between [[Hydrogen]] and [[Nitrogen]] the [[Chemical Bond|chemical bonds]] in the [[reactant]]s must be broken first before the [[Chemical Bond|bonds]] in the [[product]]s are formed. | ||
| + | |} | ||
| + | |||
| + | There are 3 H-H [[Chemical Bond|bonds]] and 1 N≡N [[Chemical Bond|bonds]]. | ||
| + | |||
| + | 3 x 436 + 1 x 941 = 2249kJ | ||
| + | |||
| + | Therefore 2249kJ/mol are needed to break the [[Chemical Bond|bonds]] in the [[reactant]]s. | ||
| + | |||
| + | There are 6 N-H [[Chemical Bond|bonds]]. | ||
| + | |||
| + | 6 x 391 = 2346kJ | ||
| + | |||
| + | Therefore 2346kJ/mol is released when the [[Chemical Bond|bonds]] in the [[product]]s form. | ||
| + | |||
| + | Once the [[Chemical Reaction|reaction]] is complete 97kJ will be released. | ||
Revision as of 16:40, 14 January 2019
Key Stage 4
Meaning
Bond Energy is the energy needed to break a chemical bond between two atoms.
About Bond Energy
- When chemical bonds are formed energy is released to the surroundings increasing the temperature. An exothermic process]].
- To break chemical bonds energy is needed to separate the atoms, decreasing the temperature of the surroundings. An endothermic process]].
Examples
Some common bond energies are given in the table below.
| Bond | Energy in kJ/mol |
| H-H | 436 |
| O=O | 498 |
| N≡N | 941 |
| C-C | 347 |
| C=C | 614 |
| C≡C | 839 |
| C-H | 413 |
| O-H | 464 |
| C=O | 799 |
| Cl-Cl | 243 |
| H-Cl | 432 |
| N-H | 391 |
These can be used to calculate the energy released per mole in a chemical reaction.
Example 1
| In the reaction between Methane and Oxygen the chemical bonds in the reactants must be broken first before the bonds in the products are formed. |
There are 4 C-H bonds and 2 O=O bonds.
4 x 413 + 2 x 498 = 2648kJ
Therefore 2648kJ/mol are needed to break the bonds in the reactants.
There are 2 C=O bonds and 4 O-H bonds.
2 x 799 + 4 x 464 = 3454kJ
Therefore 3454kJ/mol is released when the bonds in the products form.
Once the reaction is complete 806kJ will be released.
Example 2
| In the reaction between Hydrogen and Nitrogen the chemical bonds in the reactants must be broken first before the bonds in the products are formed. |
There are 3 H-H bonds and 1 N≡N bonds.
3 x 436 + 1 x 941 = 2249kJ
Therefore 2249kJ/mol are needed to break the bonds in the reactants.
There are 6 N-H bonds.
6 x 391 = 2346kJ
Therefore 2346kJ/mol is released when the bonds in the products form.
Once the reaction is complete 97kJ will be released.