Difference between revisions of "Gas"
(→Key Stage 4) |
|||
| Line 81: | Line 81: | ||
| style="height:20px; width:200px; text-align:center;" |[[Thermal Conduction]] is very poor in a [[gas]]es. | | style="height:20px; width:200px; text-align:center;" |[[Thermal Conduction]] is very poor in a [[gas]]es. | ||
|} | |} | ||
| + | |||
| + | ===Gas Volume and Mass=== | ||
Revision as of 13:17, 23 January 2019
Contents
Key Stage 2
Meaning
Gas is a state of matter that can change size and shape to fit any container.
About Gases
- Most gases are invisible but we can feel them.
- When the air moves we call it the wind.
|
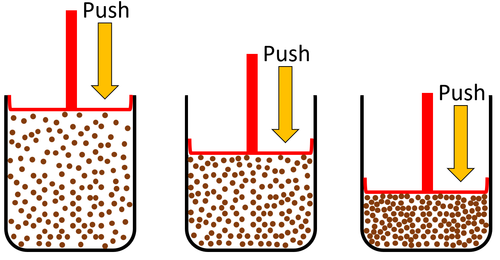
| Gases can be squashed into a smaller size. |
Examples of gas materials:
- Air (A mixture of gases, mostly nitrogen and oxygen)
- Steam
Key Stage 3
Meaning
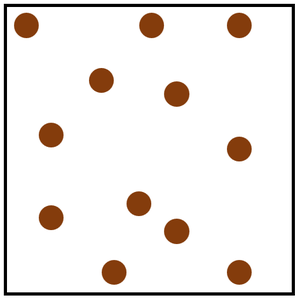
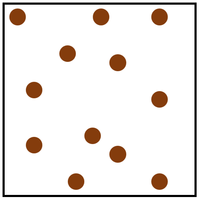
Gas is a State of Matter in which the particles are separated by large distances and can move freely.
About Gases
| Gas can be squashed into a smaller size because the particles are spread apart. |
Key Stage 4
Meaning
Gas is a State of Matter in which the particles are separated by large distances and can move freely.
About Gases
- When a substance is in its gaseous state it is always less dense than in its liquid or solid state due to the particles in a gas being spread far apart from each other.
- A substance which is gaseous at room temperature has a smaller force of attraction between particles than a substance which is liquid or solid at room temperature.
| Particle Diagram | Particle Arrangement | Property |
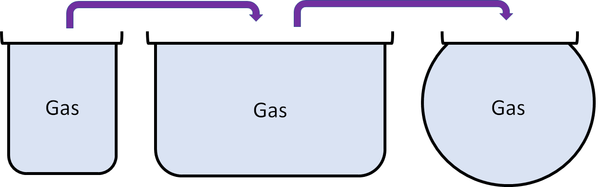
| Particles are free to move in all directions. | Gases fit the size of their container. | |
| Gases fit the shape of their container. | ||
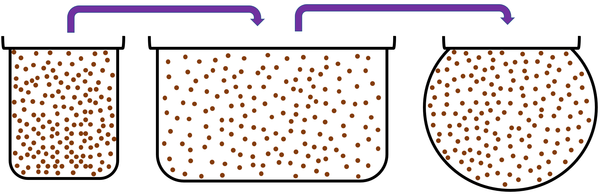
| Convection happens most easily in gases. | ||
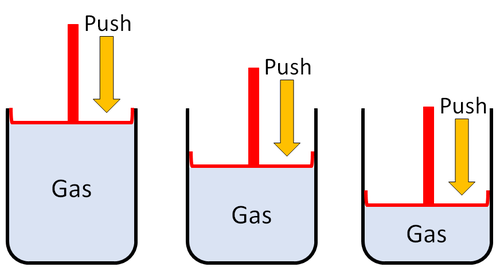
| Particles are spread apart. | Gases can be compressed into a smaller volume. | |
| Sound passes through gases slower than liquids and solids. | ||
| Thermal Conduction is very poor in a gases. |