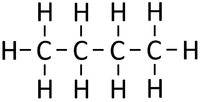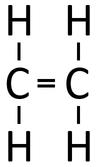Difference between revisions of "Cracking"
(→Examples) |
(→Examples) |
||
| Line 16: | Line 16: | ||
|[[File:StructuralDiagramEthane.png|center|200px]] | |[[File:StructuralDiagramEthane.png|center|200px]] | ||
|[[File:AdditionSign.png|center|100px]] | |[[File:AdditionSign.png|center|100px]] | ||
| − | |[[File:StructuralDiagramEthene.png|center| | + | |[[File:StructuralDiagramEthene.png|center|100px]] |
|- | |- | ||
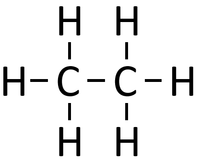
| style="height:20px; width:200px; text-align:center;" |If [[butane]] were '''cracked'''. | | style="height:20px; width:200px; text-align:center;" |If [[butane]] were '''cracked'''. | ||
Revision as of 10:18, 25 January 2019
Key Stage 4
Meaning
Cracking is a Thermal Decomposition process in which large hydrocarbon molecules are broken into smaller hydrocarbon molecules.
About Cracking
- Cracking is often done because Crude Oil contains more large hydrocarbon molecules than can be used and not enough short hydrocarbon molecules than are needed.
- When Crude Oil fractions are cracked the long alkanes are broken down into smaller alkanes and alkenes.
- Cracking is done at very high temperatures (500°C) and uses either a catalyst to aid the reaction or steam.
- When an alkane is cracked into smaller pieces there are not enough Hydrogen [[atom]s to produce two saturated hydrocarbons. One of the hydrocarbons must be unsaturated and therefor will have a double bond.
Examples
| If butane were cracked. | An alkane and an alkene are produced. | |||