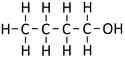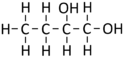Difference between revisions of "Butanol"
| (5 intermediate revisions by the same user not shown) | |||
| Line 29: | Line 29: | ||
|} | |} | ||
| − | ====Butan- | + | : The [[combustion]] of [[Butanol]] [[product|produces]] [[Carbon Dioxide]] and [[Water]]. |
| + | : [[Butanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| + | |||
| + | <math>C_4H_9OH + 6O_2 → 4CO_2 + 5H_2O</math> | ||
| + | |||
| + | : [[Butanol]] can also be [[oxidise]]d at low [[temperature]]s to [[product|produce]] [[Water]] and the [[Carboxylic Acid]]; [[Butanoic Acid]]. | ||
| + | |||
| + | <math>C_4H_9OH + O2 → C_3H_7COOH + H_2O</math> | ||
| + | |||
| + | : [[Butanol]] can be [[oxidise]]d to [[product|produce]] [[Carbon Dioxide]] and [[Water]]. | ||
| + | : [[Butanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| + | |||
| + | ====Butan-1,2-diol==== | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 39: | Line 51: | ||
| style="height:20px; width:125px; text-align:center;" |C<sub>4</sub>H<sub>8</sub>(OH)<sub>2</sub> | | style="height:20px; width:125px; text-align:center;" |C<sub>4</sub>H<sub>8</sub>(OH)<sub>2</sub> | ||
| style="height:20px; width:125px; text-align:center;" |CH<sub>3</sub>CH<sub>2</sub>CHOHCH<sub>2</sub>OH | | style="height:20px; width:125px; text-align:center;" |CH<sub>3</sub>CH<sub>2</sub>CHOHCH<sub>2</sub>OH | ||
| − | |[[File: | + | |[[File:StructuralDiagramButan12diol.png|center|125px]] |
| − | |[[File: | + | |[[File:BallandStickButan12diol.png|center|125px]] |
|} | |} | ||
| − | : [[ | + | : The [[combustion]] of '''Butandiol''' [[product|produces]] [[Carbon Dioxide]] and [[Water]]. |
| − | : | + | : '''Butandiol''' + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] |
| − | + | ||
| + | <math>2C_4H_8(OH)_2 + 11O_2 → 8CO_2 + 10H_2O</math> | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Butanol, page 238, GCSE Chemistry, CGP, AQA ''] | ||
Latest revision as of 12:20, 28 October 2019
Contents
Key Stage 3
Meaning
Butanol is a compound with chemical formula C4H9OH.
About Butanol
- Butanol is a transparent liquid (at room temperature).
- Butanol can be oxidised to produce Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Butanol is an alcohol molecule with chemical formula C4H9OH.
About Butanol
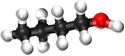
Butan-1-ol
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C4H9OH | CH3CH2CH2CH2OH |
- The combustion of Butanol produces Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
\(C_4H_9OH + 6O_2 → 4CO_2 + 5H_2O\)
- Butanol can also be oxidised at low temperatures to produce Water and the Carboxylic Acid; Butanoic Acid.
\(C_4H_9OH + O2 → C_3H_7COOH + H_2O\)
- Butanol can be oxidised to produce Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
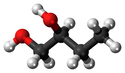
Butan-1,2-diol
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C4H8(OH)2 | CH3CH2CHOHCH2OH |
- The combustion of Butandiol produces Carbon Dioxide and Water.
- Butandiol + Oxygen → Carbon Dioxide + Water
\(2C_4H_8(OH)_2 + 11O_2 → 8CO_2 + 10H_2O\)