Difference between revisions of "Fuel Cell"
| (3 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
===About Fuel Cells=== | ===About Fuel Cells=== | ||
: In a '''fuel cell''' [[Oxygen]] is combined with [[Hydrogen]] to produce [[Water]]. | : In a '''fuel cell''' [[Oxygen]] is combined with [[Hydrogen]] to produce [[Water]]. | ||
| − | : '''Fuel cells''' are designed to combine [[Hydrogen Ion|Hydrogen ions]] and [[Hydroxide Ion|Hydroxide ions]] to produce a [[Potential Difference|potential difference]] between two [[electrode]]s. | + | : '''Fuel cells''' are designed to combine [[Hydrogen Ion (Chemistry)|Hydrogen ions]] and [[Hydroxide Ion|Hydroxide ions]] to produce a [[Potential Difference|potential difference]] between two [[electrode]]s. |
: '''Fuel cells''' may be used in electric cars and were used on the Space Shuttle. | : '''Fuel cells''' may be used in electric cars and were used on the Space Shuttle. | ||
| Line 18: | Line 18: | ||
*[[Hydrogen]] is highly [[flammable]]. | *[[Hydrogen]] is highly [[flammable]]. | ||
*[[Hydrogen]] is made by [[electrolysis]] which requires [[electricity]], which is often made by power stations burning [[fuel]] and producing [[Carbon Dioxide]]. | *[[Hydrogen]] is made by [[electrolysis]] which requires [[electricity]], which is often made by power stations burning [[fuel]] and producing [[Carbon Dioxide]]. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Fuel cell, pages 172-3, 184-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d132 ''Fuel cells, page 65, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Fuel cells, pages 122-123, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Fuel cells, pages 138-9, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Fuel cells, pages 190, 191, GCSE Chemistry, CGP, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Fuel cells, page 71, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Fuel cells, pages 124-125, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Fuel cells, pages 206, 207, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Fuel cells, page 95, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
Latest revision as of 09:21, 11 December 2019
Contents
Key Stage 4
Meaning
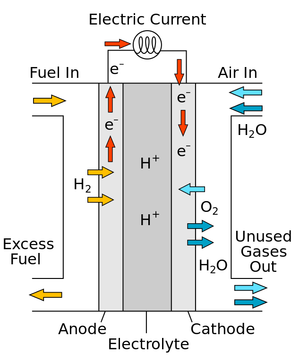
A diagram of a simple fuel cell.
A fuel cell is a device which can combine Hydrogen and Oxygen to produce a Potential Difference.
About Fuel Cells
- In a fuel cell Oxygen is combined with Hydrogen to produce Water.
- Fuel cells are designed to combine Hydrogen ions and Hydroxide ions to produce a potential difference between two electrodes.
- Fuel cells may be used in electric cars and were used on the Space Shuttle.
Advantages
- No Carbon Dioxide is produced.
- Refilling with Hydrogen is quicker than recharging a battery.
- They can be made in many different sizes for different uses.
Disadvantages
- Hydrogen must be stored as a Compressed Gas.
- Hydrogen is highly flammable.
- Hydrogen is made by electrolysis which requires electricity, which is often made by power stations burning fuel and producing Carbon Dioxide.
References
AQA
- Fuel cell, pages 172-3, 184-5, GCSE Chemistry; Student Book, Collins, AQA
- Fuel cells, page 65, GCSE Chemistry; The Revision Guide, CGP, AQA
- Fuel cells, pages 122-123, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Fuel cells, pages 138-9, GCSE Chemistry, Hodder, AQA
- Fuel cells, pages 190, 191, GCSE Chemistry, CGP, AQA
Edexcel
- Fuel cells, page 71, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Fuel cells, pages 124-125, GCSE Chemistry, Pearson, Edexcel
- Fuel cells, pages 206, 207, GCSE Chemistry, CGP, Edexcel