Difference between revisions of "Earth's Atmosphere"
(Redirected page to Earth#Earth's Atmosphere) (Tag: New redirect) |
|||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | + | ==Key Stage 3== | |
| + | ===Meaning=== | ||
| + | '''Earth's Atmosphere''' is a [[mixture]] of [[gas]]es covering the surface of the [[Earth]]. | ||
| + | |||
| + | ===About the Earth's Atmosphere=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:AtmosphericGases.png|center|400px]] | ||
| + | |- | ||
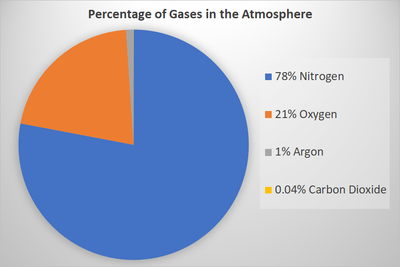
| + | | style="height:20px; width:200px; text-align:center;" |A [[Pie Chart]] showing the percentage of different [[gas]]s in the '''Earth's Atmosphere'''. | ||
| + | |} | ||
| + | |||
| + | : The '''Earth's Atmosphere''' is essential to the survival of many [[Alive|living]] creatures on the [[planet]]. | ||
| + | : The '''Earth's Atmosphere''' has not always had the same proportions of [[gas]]es. 3.9 billion [[year]]s ago there was no [[Oxygen]] in the [[atmosphere]]. In the last 200 years humans have caused the [[Carbon Dioxide]] in the '''Earth's Atmosphere''' to double. | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | '''Earth's Atmosphere''' is a [[mixture]] of [[gas]]es covering the surface of the [[Earth]]. | ||
| + | |||
| + | ===About the Earth's Atmosphere=== | ||
| + | : The '''Earth's Atmosphere''' is around 79% [[Nitrogen]], 20% [[Oxygen]] 1% [[Argon]] and 0.04% [[Carbon Dioxide]]. However, these [[percentage]]s vary depending on the [[humidity]] as [[Water Vapour]] can make up some of the '''Earth's Atmosphere'''. | ||
| + | : The '''Earth's Atmosphere''' [[Thermal Insulator|insulate]]s the [[Earth]] keeping the surface at a higher [[Mean Average|average]] [[temperature]] than if it did not have an [[atmosphere]]. | ||
| + | : The '''Earth's Atmosphere''' protects [[organism]]s on the surface from harmful [[Cosmic Ray|cosmic rays]] and solar wind. | ||
| + | |||
| + | ===Formation of the Earth's Atmosphere=== | ||
| + | : The '''Earth's Atmosphere''' formed 3.9 billion [[year]]s ago. | ||
| + | : [[Scientist]]s believe the '''Earth's Atmosphere''' was formed from [[gas]]es released from [[Volcano]]es on the early [[Earth]]. | ||
| + | : [[Gas]] from the early [[Earth]] has been trapped in small bubbles in volcanic [[rock]] showing it be mostly [[Carbon Dioxide]], [[Methane]] and [[Nitrogen]]. Some evidence also suggests there may have been high quantities of [[ammonia]]. | ||
| + | : If there had been any [[Oxygen]] in the early '''atmosphere''' it would have reacted with the [[Methane]] to produce [[Carbon Dioxide]] and [[Water]] or any [[Reactivity Series|reactive]] [[metal]]s to produce [[Metal Oxide|metal oxides]]. | ||
| + | |||
| + | ===Evolution of the Earth's Atmosphere=== | ||
| + | : Once [[organism]]s [[Evolution by Natural Selection|evolved]] which could get [[energy]] from sunlight via [[photosynthesis]] the [[Carbon Dioxide]] in the '''atmosphere''' decreased and [[Oxygen]] began to increase. | ||
| + | : [[Carbon Dioxide]] was also removed from the '''atmosphere''' when it [[dissolve]]d in the [[ocean]] and was used by some sea [[organism]]s to form hard [[Calcium Carbonate]] shells. | ||
| + | ====Extra Information==== | ||
| + | {{#ev:youtube|https://www.youtube.com/watch?v=dO2xx-aeZ4w}} | ||
| + | |||
| + | ===Equilibrium of the Earth's Atmosphere=== | ||
| + | : The amount of different [[gas]]es in the '''atmosphere''' have remained roughly the same for the last 3 million years due to a balance of processes which either add [[gas]]es to the '''atmosphere''' or remove it, including the [[Carbon Cycle|carbon cycle]] and the [[Nitrogen Cycle|nitrogen cycle (higher tier)]]. | ||
| + | : [[Carbon Dioxide]] is constantly produced by [[volcano]]es, [[combustion]] and [[respiration]], but it is removed from the [[atmosphere]] by [[photosynthesis]] and [[dissolve|dissolving]] in the [[ocean]]. | ||
| + | : [[Oxygen]] is constantly produced by [[photosynthesis]] but removed by [[combustion]] and [[respiration]]. | ||
| + | |||
| + | ===Human effects on the Atmosphere=== | ||
| + | : The equilibrium of [[gas]]es has been affected by [[combustion]] and other industrial processes caused by [[human]]s. | ||
| + | : Before the industrial revolution the amount of [[Carbon Dioxide]] in the '''atmosphere''' was 0.02%. Since then, due to the [[combustion]] of [[Fossil Fuel|fossil fuels]] it has increased to 0.04% which [[scientist]]s believe is causing the [[Mean Average|average]] global [[temperature]] to increase. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09697 ''Atmosphere, page 155, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d46 ''Atmosphere, page 91, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Atmosphere, pages 166, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere, pages 194-205, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Atmosphere, pages 206-213, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Atmosphere, pages 268-275, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Atmosphere, pages 292-3, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; ammonia, page 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Atmosphere; carbon dioxide levels, pages 228-9, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Atmosphere; carbon dioxide removal, pages 226-7, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; carbon dioxide, pages 194-196, 198-201, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Atmosphere; Composition today, pages 167, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; composition, page 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Atmosphere; Early composition, pages 167-8, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Atmosphere; early composition, pages 224-5, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; early Earth, page 194, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Atmosphere; early, pages 296-7, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; evolution, pages 196-197, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Atmosphere; Greenhouse gases, pages 170-3, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; greenhouse gases, pages 198-201, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Atmosphere; Methane levels, pages 229-30, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; methane, pages 194, 197, 198, 201, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Atmosphere; Origin of oxygen, pages 225-6, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; oxygen, pages 194-195, 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; photosynthesis, pages 194-195, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Atmosphere; Pollutants, pages 179-81, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Atmosphere; pollutants, pages 202-203, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Atmosphere; Present composition, page 224, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Atmosphere; Removal of carbon dioxide, pages 169-70, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Atmosphere; Source of oxygen, pages 168-9, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Atmosphere; Sources of pollution, pages 237-8, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Atmospheric pressure, page 120-1, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Atmospheric pressure, page 139, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Atmospheric pressure, page 171, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Atmospheric pressure, pages 166-167, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Atmospheric pressure, pages 174-5, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac23 ''Atmospheric pressure, pages 41, 59, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | ====Edexcel==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Atmosphere, pages 141, 143, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Atmosphere, pages 264-269, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Atmosphere, pages 91-93, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Atmosphere; changing, pages 164-165, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Atmosphere; changing, pages 278-279, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Atmosphere; composition, page 162, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Atmosphere; composition, page 276, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Atmosphere; early, pages 162-163, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Atmosphere; early, pages 276-277, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Atmosphere; present-day, pages 166-167, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Atmosphere; present-day, pages 280-281, GCSE Combined Science, Pearson Edexcel ''] | ||
Latest revision as of 11:34, 29 October 2019
Contents
Key Stage 3
Meaning
Earth's Atmosphere is a mixture of gases covering the surface of the Earth.
About the Earth's Atmosphere
| A Pie Chart showing the percentage of different gass in the Earth's Atmosphere. |
- The Earth's Atmosphere is essential to the survival of many living creatures on the planet.
- The Earth's Atmosphere has not always had the same proportions of gases. 3.9 billion years ago there was no Oxygen in the atmosphere. In the last 200 years humans have caused the Carbon Dioxide in the Earth's Atmosphere to double.
Key Stage 4
Meaning
Earth's Atmosphere is a mixture of gases covering the surface of the Earth.
About the Earth's Atmosphere
- The Earth's Atmosphere is around 79% Nitrogen, 20% Oxygen 1% Argon and 0.04% Carbon Dioxide. However, these percentages vary depending on the humidity as Water Vapour can make up some of the Earth's Atmosphere.
- The Earth's Atmosphere insulates the Earth keeping the surface at a higher average temperature than if it did not have an atmosphere.
- The Earth's Atmosphere protects organisms on the surface from harmful cosmic rays and solar wind.
Formation of the Earth's Atmosphere
- The Earth's Atmosphere formed 3.9 billion years ago.
- Scientists believe the Earth's Atmosphere was formed from gases released from Volcanoes on the early Earth.
- Gas from the early Earth has been trapped in small bubbles in volcanic rock showing it be mostly Carbon Dioxide, Methane and Nitrogen. Some evidence also suggests there may have been high quantities of ammonia.
- If there had been any Oxygen in the early atmosphere it would have reacted with the Methane to produce Carbon Dioxide and Water or any reactive metals to produce metal oxides.
Evolution of the Earth's Atmosphere
- Once organisms evolved which could get energy from sunlight via photosynthesis the Carbon Dioxide in the atmosphere decreased and Oxygen began to increase.
- Carbon Dioxide was also removed from the atmosphere when it dissolved in the ocean and was used by some sea organisms to form hard Calcium Carbonate shells.
Extra Information
Equilibrium of the Earth's Atmosphere
- The amount of different gases in the atmosphere have remained roughly the same for the last 3 million years due to a balance of processes which either add gases to the atmosphere or remove it, including the carbon cycle and the nitrogen cycle (higher tier).
- Carbon Dioxide is constantly produced by volcanoes, combustion and respiration, but it is removed from the atmosphere by photosynthesis and dissolving in the ocean.
- Oxygen is constantly produced by photosynthesis but removed by combustion and respiration.
Human effects on the Atmosphere
- The equilibrium of gases has been affected by combustion and other industrial processes caused by humans.
- Before the industrial revolution the amount of Carbon Dioxide in the atmosphere was 0.02%. Since then, due to the combustion of fossil fuels it has increased to 0.04% which scientists believe is causing the average global temperature to increase.
References
AQA
- Atmosphere, page 155, GCSE Combined Science; The Revision Guide, CGP, AQA
- Atmosphere, page 91, GCSE Chemistry; The Revision Guide, CGP, AQA
- Atmosphere, pages 166, GCSE Combined Science Trilogy 2, Hodder, AQA
- Atmosphere, pages 194-205, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere, pages 206-213, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Atmosphere, pages 268-275, GCSE Chemistry, CGP, AQA
- Atmosphere, pages 292-3, GCSE Chemistry; Student Book, Collins, AQA
- Atmosphere; ammonia, page 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; carbon dioxide levels, pages 228-9, GCSE Chemistry, Hodder, AQA
- Atmosphere; carbon dioxide removal, pages 226-7, GCSE Chemistry, Hodder, AQA
- Atmosphere; carbon dioxide, pages 194-196, 198-201, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; Composition today, pages 167, GCSE Combined Science Trilogy 2, Hodder, AQA
- Atmosphere; composition, page 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; Early composition, pages 167-8, GCSE Combined Science Trilogy 2, Hodder, AQA
- Atmosphere; early composition, pages 224-5, GCSE Chemistry, Hodder, AQA
- Atmosphere; early Earth, page 194, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; early, pages 296-7, GCSE Chemistry; Student Book, Collins, AQA
- Atmosphere; evolution, pages 196-197, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; Greenhouse gases, pages 170-3, GCSE Combined Science Trilogy 2, Hodder, AQA
- Atmosphere; greenhouse gases, pages 198-201, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; Methane levels, pages 229-30, GCSE Chemistry, Hodder, AQA
- Atmosphere; methane, pages 194, 197, 198, 201, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; Origin of oxygen, pages 225-6, GCSE Chemistry, Hodder, AQA
- Atmosphere; oxygen, pages 194-195, 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; photosynthesis, pages 194-195, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; Pollutants, pages 179-81, GCSE Combined Science Trilogy 2, Hodder, AQA
- Atmosphere; pollutants, pages 202-203, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Atmosphere; Present composition, page 224, GCSE Chemistry, Hodder, AQA
- Atmosphere; Removal of carbon dioxide, pages 169-70, GCSE Combined Science Trilogy 2, Hodder, AQA
- Atmosphere; Source of oxygen, pages 168-9, GCSE Combined Science Trilogy 2, Hodder, AQA
- Atmosphere; Sources of pollution, pages 237-8, GCSE Chemistry, Hodder, AQA
- Atmospheric pressure, page 120-1, GCSE Chemistry; Student Book, Collins, AQA
- Atmospheric pressure, page 139, GCSE Physics, Hodder, AQA
- Atmospheric pressure, page 171, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Atmospheric pressure, pages 166-167, GCSE Physics; Third Edition, Oxford University Press, AQA
- Atmospheric pressure, pages 174-5, GCSE Physics; Student Book, Collins, AQA
- Atmospheric pressure, pages 41, 59, GCSE Physics; The Revision Guide, CGP, AQA
Edexcel
- Atmosphere, pages 141, 143, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Atmosphere, pages 264-269, GCSE Chemistry, CGP, Edexcel
- Atmosphere, pages 91-93, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Atmosphere; changing, pages 164-165, GCSE Chemistry, Pearson, Edexcel
- Atmosphere; changing, pages 278-279, GCSE Combined Science, Pearson Edexcel
- Atmosphere; composition, page 162, GCSE Chemistry, Pearson, Edexcel
- Atmosphere; composition, page 276, GCSE Combined Science, Pearson Edexcel
- Atmosphere; early, pages 162-163, GCSE Chemistry, Pearson, Edexcel
- Atmosphere; early, pages 276-277, GCSE Combined Science, Pearson Edexcel
- Atmosphere; present-day, pages 166-167, GCSE Chemistry, Pearson, Edexcel
- Atmosphere; present-day, pages 280-281, GCSE Combined Science, Pearson Edexcel