Difference between revisions of "Methanol"
(→About Methanol) |
|||
| Line 31: | Line 31: | ||
: [[Methanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | : [[Methanol]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
: <chem>2CH3OH + 3O2 -> 2CO2 + 4H2O</chem> | : <chem>2CH3OH + 3O2 -> 2CO2 + 4H2O</chem> | ||
| − | : [[ | + | : [[Methanol]] can also be [[oxidise]]d at low [[temperature]]s to [[product|produce]] [[Water]] and the [[Carboxylic Acid]]; [[Methanoic Acid]]. |
: <chem>CH3OH + O2 -> CHOOH + H2O</chem> | : <chem>CH3OH + O2 -> CHOOH + H2O</chem> | ||
Revision as of 07:15, 24 April 2019
Key Stage 3
Meaning
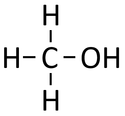
Methanol is a compound with chemical formula CH3OH.
About Methanol
- Methanol is a transparent liquid (at room temperature).
- Methanol can be oxidised to produce Carbon Dioxide and Water.
- Methanol + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Methanol is an alcohol molecule with chemical formula CH3OH.
About Methanol
- Methanol is a transparent liquid (at STP).
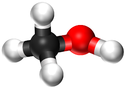
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| CH3OH | CH3OH |
- The combustion of methanol produces Carbon Dioxide and Water.
- Methanol + Oxygen → Carbon Dioxide + Water
- <chem>2CH3OH + 3O2 -> 2CO2 + 4H2O</chem>
- Methanol can also be oxidised at low temperatures to produce Water and the Carboxylic Acid; Methanoic Acid.
- <chem>CH3OH + O2 -> CHOOH + H2O</chem>