Difference between revisions of "Conservation of Mass"
(→Calculating the Mass of a missing Product/Reactant) |
(→Calculating the Mass of a missing Product/Reactant) |
||
| Line 52: | Line 52: | ||
===Calculating the Mass of a missing Product/Reactant=== | ===Calculating the Mass of a missing Product/Reactant=== | ||
| + | : The [[mass]] of a missing [[product]] of [[reactant]] can be found because the total [[mass]] of the [[product]]s = the total [[mass]] of [[reactant]]s. | ||
| + | M<sub>Reactants</sub> = M<sub>Products</sub> | ||
| + | {| class="wikitable" | ||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | Find the [[mass]] of [[Calcium Oxide]] [[product|produced]] in the following [[Chemical Reaction|reaction]]: | ||
| + | |||
| + | CaCO<sub>3</sub> → CaO + CO<sub>2</sub> | ||
| + | |||
| + | 25g = x + 11g | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | Find the [[mass]] of [[Carbon Dioxide]] [[product|produced]] in the following [[Chemical Reaction|reaction]]: | ||
| + | |||
| + | CH<sub>4</sub> + 2O<sub>2</sub> → 2H<sub>2</sub>O + CO<sub>2</sub> | ||
| + | |||
| + | 4g + 16g = 9g + x | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | Find the [[mass]] of [[Hydrochloric Acid]] needed in the following [[Chemical Reaction|reaction]]: | ||
| + | |||
| + | NaOH + HCl → NaCl + H<sub>2</sub>O | ||
| + | |||
| + | 160g + x = 234g + 72g | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | x = 25g - 11g | ||
| + | |||
| + | x = 14g | ||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | 20g = 9g + x | ||
| + | |||
| + | x = 20g - 9g | ||
| + | |||
| + | x = 11g | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | 160g + x = 306g | ||
| + | |||
| + | x = 306g - 160g | ||
| + | |||
| + | x = 146g | ||
| + | |} | ||
| + | |||
| + | ===Calculating the Mass Required for a Complete Reaction=== | ||
{| class="wikitable" | {| class="wikitable" | ||
| style="height:20px; width:200px; text-align:center;" | | | style="height:20px; width:200px; text-align:center;" | | ||
Revision as of 12:10, 3 January 2019
Contents
Key Stage 3
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
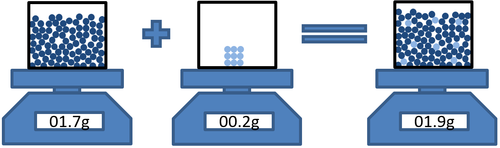
- In dissolving conservation of mass means that the mass of the solvent and the mass of the solute can be added to find the mass of the solution.
| Masssolvent + Masssolute = Masssolution |
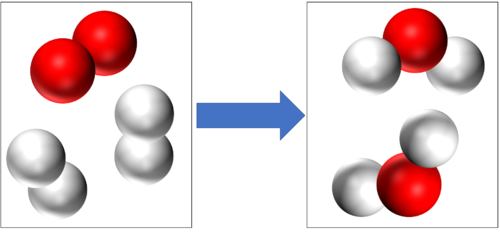
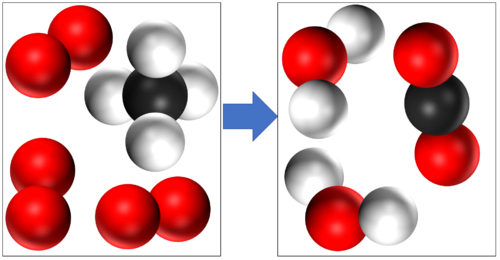
- In a chemical reaction conservation of mass means that the same atoms which made up the reactants must make up the products. So the atoms are not created or destroyed in a chemical reaction, they are just rearranged.
|
Conservation of mass tells us that if there are 4 Hydrogen atoms and 2 Oxygen atoms at the start of this reaction then there will be the end of the reaction 4 Hydrogen atoms and 2 Oxygen atoms at the end of the reaction. |
|
In this reaction you can see that mass is conserved because there are 4 Hydrogen atoms, 4 Oxygen atoms and 1 Carbon atom in the reactants and all the same atoms are found in the products. |
Key Stage 4
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
- In a chemical reaction law of conservation of mass indicates that the total mass of the products is the same as the total mass of the reactants.
Examples
Methane + Oxygen → Water + Carbon Dioxide
CH4 + 2O2 → 2H2O + CO2
16g + 64g = 36g + 44g
Sodium Hydroxide + Hydrochloric Acid → Sodium Chloride + Water
NaOH + HCl → NaCl + H2O
40g + 36.5g = 58.5 + 18g
Calculating the Mass of a missing Product/Reactant
- The mass of a missing product of reactant can be found because the total mass of the products = the total mass of reactants.
MReactants = MProducts
|
Find the mass of Calcium Oxide produced in the following reaction: CaCO3 → CaO + CO2 25g = x + 11g |
Find the mass of Carbon Dioxide produced in the following reaction: CH4 + 2O2 → 2H2O + CO2 4g + 16g = 9g + x |
Find the mass of Hydrochloric Acid needed in the following reaction: NaOH + HCl → NaCl + H2O 160g + x = 234g + 72g |
|
x = 25g - 11g x = 14g |
20g = 9g + x x = 20g - 9g x = 11g |
160g + x = 306g x = 306g - 160g x = 146g |
Calculating the Mass Required for a Complete Reaction
|
Find the mass of Calcium Oxide produced in the following reaction: CaCO3 → CaO + CO2 25g = x + 11g |
Find the mass of Carbon Dioxide produced in the following reaction: CH4 + 2O2 → 2H2O + CO2 4g + 16g = 9g + x |
Find the mass of Hydrochloric Acid needed in the following reaction: NaOH + HCl → NaCl + H2O 160g + x = 234g + 72g |
|
x = 25g - 11g x = 14g |
20g = 9g + x x = 20g - 9g x = 11g |
160g + x = 306g x = 306g - 160g x = 146g |