Difference between revisions of "Rate of Reaction"
(→Examples) |
|||
| Line 25: | Line 25: | ||
====Continuously Measuring Volume of Gas==== | ====Continuously Measuring Volume of Gas==== | ||
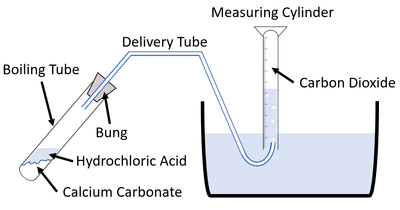
| − | + | For [[reaction]]s which give off a [[gas]] the [[Volume (Space)|volume]] of [[gas]] [[product|produced]] can be [[measure]]d throughout an [[experiment]] to find the '''rate of reaction'''. | |
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:MeasuringGasGivenOff.png|center|400px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |This [[diagram]] shows a possible setup for [[measuring]] the [[gas]] given off during an [[experiment]]. | ||
| + | |} | ||
====Continuously Measuring the Mass==== | ====Continuously Measuring the Mass==== | ||
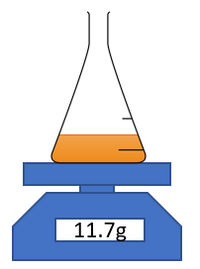
| − | For [[reaction]]s which give off a gas | + | For [[reaction]]s which give off a [[gas]] the [[mass]] of [[Reaction Mixture|reaction mixture]] can be [[measure]]d throughout an [[experiment]] to find the '''rate of reaction'''. |
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:MeasuringMass.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |This [[diagram]] shows a possible setup for [[measuring]] the [[mass]] of the [[Reaction Mixture|reaction mixture]] during an [[experiment]]. | ||
| + | |} | ||
| + | ====Continuously Measuring the Opacity==== | ||
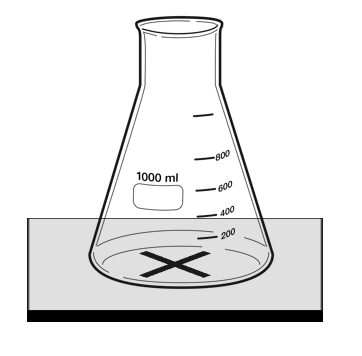
| + | For [[reaction]]s in which the [[reactant]]s are in a [[transparent]] [[solution]] but the [[product]]s form an [[insoluble]] [[precipitate]] that is [[opaque]] then the [[opacity]] can be [[measure]]d. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:MeasuringOpacity.png|center|400px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |This [[diagram]] shows a possible setup for identifying when a [[mixture]] becomes [[opaque]] during a [[Chemical Reaction|reaction]]. | ||
| + | |} | ||
Revision as of 12:08, 16 January 2019
Contents
Key Stage 4
Meaning
Rate of reaction is a measure of how quickly the reactants react to create the products.
About the Rate of Reaction
- The longer the time taken for a reaction the lower the rate of reaction. The shorter the time taken for a reaction the higher the rate of reaction.
- High rates of reaction are important to in industries where a lot of products are needed in a short amount of time. This can save money.
- Low rates of reaction are important in materials that corrode with chemicals in the environment. This allows them to last a long time before destroyed by Oxidation or chemical weathering.
Examples
| Rusting has a low rate of reaction. | Burning Magnesium ribbon has a high rate of reaction. |
Determining the Rate of Reaction
There are two approaches to finding the rate of reaction for some chemicals.
Continuously Measuring Volume of Gas
For reactions which give off a gas the volume of gas produced can be measured throughout an experiment to find the rate of reaction.
| This diagram shows a possible setup for measuring the gas given off during an experiment. |
Continuously Measuring the Mass
For reactions which give off a gas the mass of reaction mixture can be measured throughout an experiment to find the rate of reaction.
| This diagram shows a possible setup for measuring the mass of the reaction mixture during an experiment. |
Continuously Measuring the Opacity
For reactions in which the reactants are in a transparent solution but the products form an insoluble precipitate that is opaque then the opacity can be measured.
| This diagram shows a possible setup for identifying when a mixture becomes opaque during a reaction. |