Difference between revisions of "Tritium"
| Line 13: | Line 13: | ||
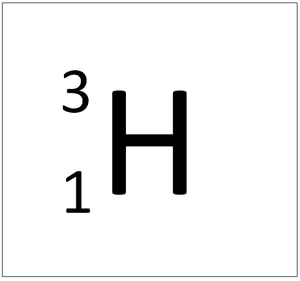
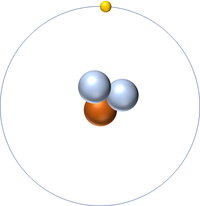
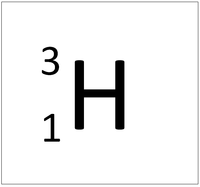
| style="height:20px; width:200px; text-align:center;" colspan = "2"|[[Hydrogen]] always has 1 [[proton]] but in this [[isotope]] there is 2 [[neutron]]s. This [[isotope]] of [[Hydrogen]] is known as [[Tritium]]. | | style="height:20px; width:200px; text-align:center;" colspan = "2"|[[Hydrogen]] always has 1 [[proton]] but in this [[isotope]] there is 2 [[neutron]]s. This [[isotope]] of [[Hydrogen]] is known as [[Tritium]]. | ||
|} | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Tritium, pages 27, 100, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
Latest revision as of 12:59, 14 November 2019
Key Stage 4
Meaning
Tritium is an isotope of Hydrogen containing two neutrons.
About Tritium
- Tritium has all the same chemical properties of Hydrogen.
- Tritium is an unstable isotope with a half life of around 12.3 years.
| Hydrogen always has 1 proton but in this isotope there is 2 neutrons. This isotope of Hydrogen is known as Tritium. | |