Difference between revisions of "Conservation of Mass"
(Created page with "==Key Stage 3== ===Meaning=== Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to an...") |
|||
| Line 5: | Line 5: | ||
===About Conservation of Mass=== | ===About Conservation of Mass=== | ||
: In a [[Chemical Reaction|chemical reaction]] '''conservation of mass''' means that the same [[atom]]s with made up the [[reactant]]s must make up the [[product]]s. So the [[atom]]s are not created or destroyed in a [[Chemical Reaction|chemical reaction]], they are just rearranged. | : In a [[Chemical Reaction|chemical reaction]] '''conservation of mass''' means that the same [[atom]]s with made up the [[reactant]]s must make up the [[product]]s. So the [[atom]]s are not created or destroyed in a [[Chemical Reaction|chemical reaction]], they are just rearranged. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:RearrangeAtoms1.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:500px; text-align:center;" | | ||
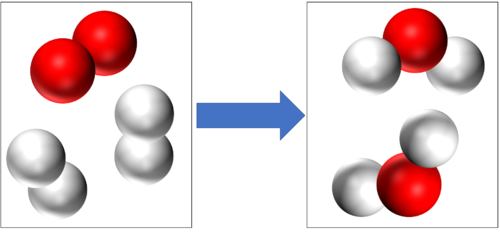
| + | '''Conservation of mass''' tells us that if there are 4 [[Hydrogen]] [[atom]]s and 2 [[Oxygen]] [[atom]]s at the start of this [[Chemical Reaction|reaction]] then there will be the end of the [[Chemical Reaction|reaction]] 4 [[Hydrogen]] [[atom]]s and 2 [[Oxygen]] [[atom]]s at the end of the [[Chemical Reaction|reaction]]. | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:RearrangeAtoms2.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:500px; text-align:center;" | | ||
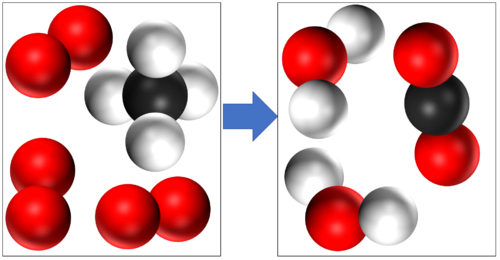
| + | In this [[Chemical Reaction|reaction]] you can see that '''mass is conserved''' because there are 4 [[Hydrogen]] [[atom]]s, 4 [[Oxygen]] [[atom]]s and 1 [[Carbon]] [[atom]] in the [[reactant]]s and all the same [[atom]]s are found in the [[product]]s. | ||
| + | |} | ||
Revision as of 20:06, 23 September 2018
Key Stage 3
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
- In a chemical reaction conservation of mass means that the same atoms with made up the reactants must make up the products. So the atoms are not created or destroyed in a chemical reaction, they are just rearranged.
|
Conservation of mass tells us that if there are 4 Hydrogen atoms and 2 Oxygen atoms at the start of this reaction then there will be the end of the reaction 4 Hydrogen atoms and 2 Oxygen atoms at the end of the reaction. |
|
In this reaction you can see that mass is conserved because there are 4 Hydrogen atoms, 4 Oxygen atoms and 1 Carbon atom in the reactants and all the same atoms are found in the products. |