Conservation of Mass
Key Stage 3
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
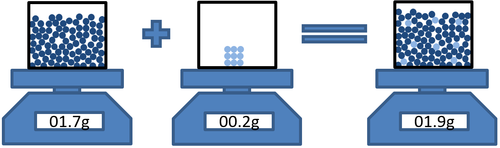
- In dissolving conservation of mass means that the mass of the solvent and the mass of the solute can be added to find the mass of the solution.
| Masssolvent + Masssolute = Masssolution |
- In a chemical reaction conservation of mass means that the same atoms which made up the reactants must make up the products. So the atoms are not created or destroyed in a chemical reaction, they are just rearranged.
|
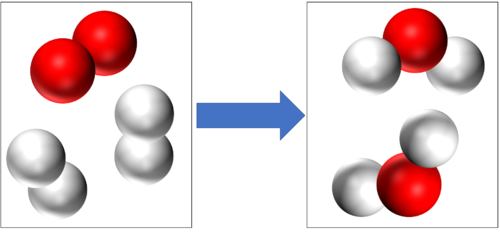
Conservation of mass tells us that if there are 4 Hydrogen atoms and 2 Oxygen atoms at the start of this reaction then there will be the end of the reaction 4 Hydrogen atoms and 2 Oxygen atoms at the end of the reaction. |
|
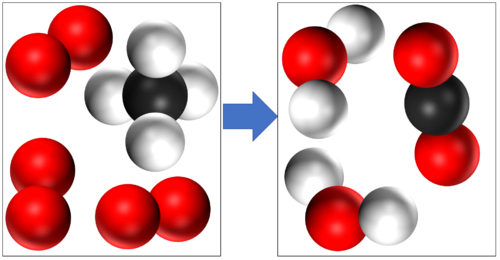
In this reaction you can see that mass is conserved because there are 4 Hydrogen atoms, 4 Oxygen atoms and 1 Carbon atom in the reactants and all the same atoms are found in the products. |
Key Stage 4
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
- In a chemical reaction law of conservation of mass indicates that the total mass of the products is the same as the total mass of the reactants.
- Conservation of mass can be observed in closed systems in which none of the products can escape and no other chemicals enter the system.
- In an open system any chemical reaction which produces a gas will appear to decrease in mass but only because the mass has moved to a different location. The particles of gas escape the container.
Examples
Methane + Oxygen → Water + Carbon Dioxide
CH4 + 2O2 → 2H2O + CO2
16g + 64g = 36g + 44g
Sodium Hydroxide + Hydrochloric Acid → Sodium Chloride + Water
NaOH + HCl → NaCl + H2O
40g + 36.5g = 58.5 + 18g
Calculating the Mass of a missing Product/Reactant
- The mass of a missing product of reactant can be found because the total mass of the products = the total mass of reactants.
MReactants = MProducts
|
Find the mass of Calcium Oxide produced in the following reaction: CaCO3 → CaO + CO2 25g = x + 11g |
Find the mass of Carbon Dioxide produced in the following reaction: CH4 + 2O2 → 2H2O + CO2 4g + 16g = 9g + x |
Find the mass of Hydrochloric Acid needed in the following reaction: NaOH + HCl → NaCl + H2O 160g + x = 234g + 72g |
|
x = 25g - 11g x = 14g |
20g = 9g + x x = 20g - 9g x = 11g |
160g + x = 306g x = 306g - 160g x = 146g |
Calculating the Mass Required for a Complete Reaction
|
Find the mass of Oxygen needed to completely oxidise all of the Magnesium: 2Mg + O2 → 2MgO 48g + x = y |
Find the mass of Oxygen needed for the complete combustion of Methane. CH4 + 2O2 → 2H2O + CO2 32g + x = y |
Find the mass of Hydrochloric Acid needed to completely neutralise all of the Sodium Hydroxide. NaOH + HCl → NaCl + H2O 20g + x = y + z |
|
Find the Relative Formula Mass of the reactants. Mr of Mg = 24g Mr of O2 = 16x2 Mr of O2 = 32g |
Find the Relative Formula Mass of the reactants. Mr of CH4 = 16g Mr of O2 = 16x2 Mr of O2 = 32g |
Find the Relative Formula Mass of the reactants. Mr of NaOH = 40g Mr of HCl = 36.5g |
|
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{48}{24}\) No. Moles = 2 Mole Therefore 1 mole of O2 is needed. 1 mole of O2 = 32g |
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{32}{16}\) No. Moles = 2 Mole Therefore 4 moles of O2 are needed. 4 moles of O2 = 128g |
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{20}{40}\) No. Moles = 0.5 Mole Therefore 0.5 mole of HCl is needed. 0.5 mole of HCl = 18.25g |
Beyond the Curriculum
References
AQA
- Conservation of mass, page 181, GCSE Combined Science Trilogy 1, Hodder, AQA
- Conservation of mass, page 43, GCSE Chemistry; The Revision Guide, CGP, AQA
- Conservation of mass, page 6, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Conservation of mass, page 68, GCSE Chemistry, Hodder, AQA
- Conservation of mass, page 78, GCSE Physics; Third Edition, Oxford University Press, AQA
- Conservation of mass, pages 108-110, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Conservation of mass, pages 114- 116, GCSE Chemistry, CGP, AQA
- Conservation of mass, pages 124, 125, 195, GCSE Combined Science; The Revision Guide, CGP, AQA
- Conservation; of mass, pages 88, 116-17, GCSE Physics; Student Book, Collins, AQA
Edexcel
- Conservation of mass, apge 26, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Conservation of mass, law of, pages 218-219, GCSE Combined Science, Pearson Edexcel
- Conservation of mass, law of, pages 74-75, GCSE Chemistry, Pearson, Edexcel
- Conservation of mass, page 89, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Conservation of mass, pages 74-76, GCSE Chemistry, CGP, Edexcel
- Mass conservation, pages 74-75, GCSE Chemistry, Pearson, Edexcel