Difference between revisions of "Solid"
| Line 58: | Line 58: | ||
===About Solids=== | ===About Solids=== | ||
| − | : When a [[substance]] is in its [[solid]] [[ | + | : When a [[substance]] is in its [[solid]] [[State of Matter|state]] it is usually more [[Density|dense]] than in its [[liquid]] or [[gas]]eous [[State of Matter|state]]. [[Ice]] is an exception to this due to how the [[molecule]]s arrange themselves, see [[Ice-Water Anomaly]]. |
: A [[substance]] which is [[solid]] at [[Room Temperature|room temperature]] has a larger [[force]] of [[attraction]] between [[particle]]s than a [[substance]] which is [[liquid]] or [[gas]] at [[Room Temperature|room temperature]]. | : A [[substance]] which is [[solid]] at [[Room Temperature|room temperature]] has a larger [[force]] of [[attraction]] between [[particle]]s than a [[substance]] which is [[liquid]] or [[gas]] at [[Room Temperature|room temperature]]. | ||
Revision as of 18:01, 23 December 2018
Contents
Key Stage 2
Meaning
Solid is a state of matter that holds its shape and cannot be squashed into a smaller space.
About Solids
- Solids can be described with texture.
|
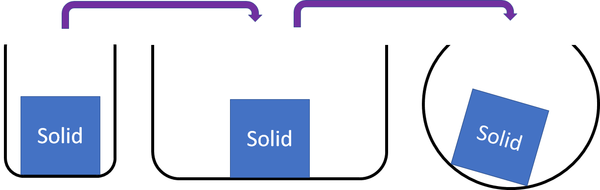
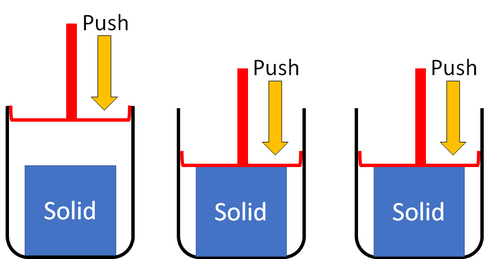
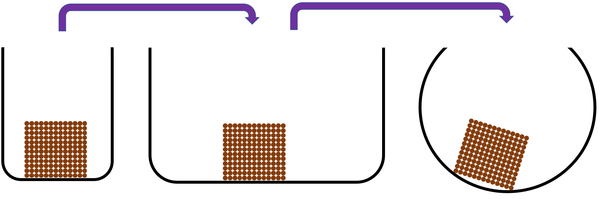
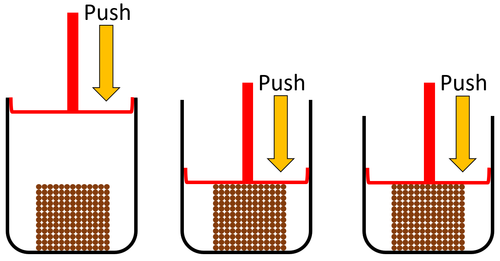
| Solids cannot be squashed into a smaller size. You can change their shape by squashing, but their size stays the same. |
Examples of solid materials:
- Brick
- Wood
- Plastic
- Glass
- Ice
Key Stage 3
Meaning
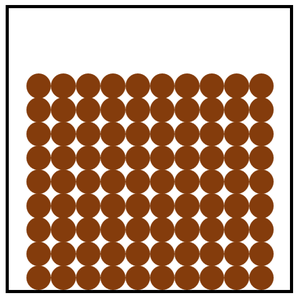
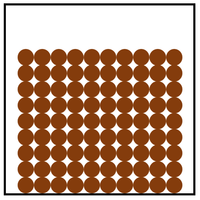
Solid is a state of matter where all the particles in fixed positions, are touching and are in a regular arrangement.
About Solids
| Solids cannot be squashed into a smaller size because the particles are already touching so they can't get any closer together. |
Key Stage 4
Meaning
Solid is a state of matter where all the particles vibrate around fixed positions.
About Solids
- When a substance is in its solid state it is usually more dense than in its liquid or gaseous state. Ice is an exception to this due to how the molecules arrange themselves, see Ice-Water Anomaly.
- A substance which is solid at room temperature has a larger force of attraction between particles than a substance which is liquid or gas at room temperature.
| Particle Diagram | Particle Arrangement | Property |
| Particles are in fixed positions. | Solids hold their shape. | |
| Convection cannot happen in solids. | ||
| Particles are very close together. | Solids cannot be compressed. | |
| Sound passes through solids faster than liquids and gases. | ||
| Particles vibrate. | ||
| Thermal Conduction happens best in solids. |