Contents
Key Stage 3
Meaning
Diffusion is when particles spread from a region of high concentration to a region of low concentration.
About Diffusion
- Diffusion can only happen in a fluid (a liquid or a gas) because the particles can move past each other.
- Diffusion cannot happen in a solid because the particles are held in fixed positions.
- In diffusion the particles always spread from a high concentration (where there are lots of the particles, to a low concentration (where there are less of the particles).
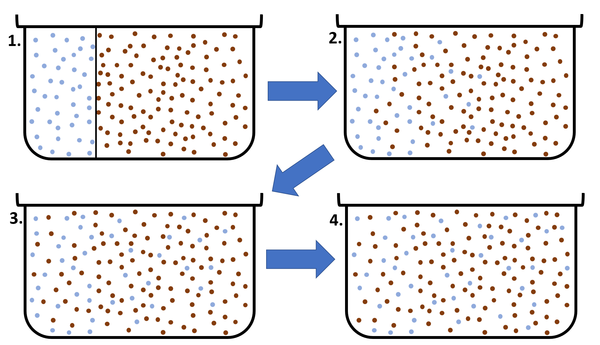
| This diagram shows a high concentration of blue particles on the left separated from the red particles by a barrier. When the barrier is removed the blue particles diffuse to the area of lower concentration on the right. Diffusion continues until all particles are equally spread. |
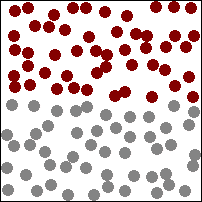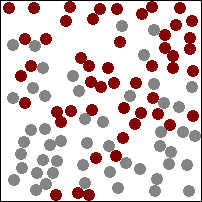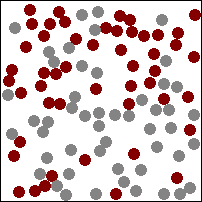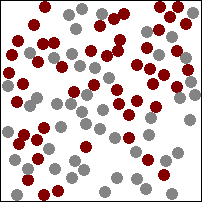
| This animation shows a high concentration of red particles initially at the top. When diffusion begins the red particles spread to the area of lower concentration at the bottom. Diffusion continues until all particles are equally spread. |
Key Stage 4
Meaning
Diffusion is when particles spread from a region of high concentration to a region of low concentration along a concentration gradient.
About Diffusion
- Diffusion can only happen in a fluid (a liquid or a gas) because the particles can move past each other.
- Diffusion cannot happen in a solid because the particles are held in fixed positions.
- In diffusion the particles always follow the concentration gradient spreading from a high concentration (where there is lots of the particles), to a low concentration (where there are less of the particles).
- Diffusion stops when all substances are spread out equally and there is no longer a concentration gradient.
| This diagram shows a high concentration of blue particles on the left separated from the red particles by a barrier. When the barrier is removed the blue particles diffuse to the area of lower concentration on the right. Diffusion continues until all particles are equally spread. |
| This animation shows a high concentration of red particles initially at the top. When diffusion begins the red particles spread to the area of lower concentration at the bottom. Diffusion continues until all particles are equally spread. |