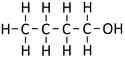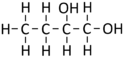Difference between revisions of "Butanol"
| Line 59: | Line 59: | ||
<math>2C_4H_8(OH)_2 + 11O_2 → 8CO_2 + 10H_2O</math> | <math>2C_4H_8(OH)_2 + 11O_2 → 8CO_2 + 10H_2O</math> | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Butanol, page 238, GCSE Chemistry, CGP, AQA ''] | ||
Latest revision as of 12:20, 28 October 2019
Contents
Key Stage 3
Meaning
Butanol is a compound with chemical formula C4H9OH.
About Butanol
- Butanol is a transparent liquid (at room temperature).
- Butanol can be oxidised to produce Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Butanol is an alcohol molecule with chemical formula C4H9OH.
About Butanol
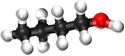
Butan-1-ol
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C4H9OH | CH3CH2CH2CH2OH |
- The combustion of Butanol produces Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
\(C_4H_9OH + 6O_2 → 4CO_2 + 5H_2O\)
- Butanol can also be oxidised at low temperatures to produce Water and the Carboxylic Acid; Butanoic Acid.
\(C_4H_9OH + O2 → C_3H_7COOH + H_2O\)
- Butanol can be oxidised to produce Carbon Dioxide and Water.
- Butanol + Oxygen → Carbon Dioxide + Water
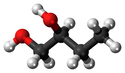
Butan-1,2-diol
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C4H8(OH)2 | CH3CH2CHOHCH2OH |
- The combustion of Butandiol produces Carbon Dioxide and Water.
- Butandiol + Oxygen → Carbon Dioxide + Water
\(2C_4H_8(OH)_2 + 11O_2 → 8CO_2 + 10H_2O\)