Difference between revisions of "Density"
| (31 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | The amount of [[mass]] per [[unit]] [[volume]] of an [[object]]. | + | ==Key Stage 3== |
| + | ===Meaning=== | ||
| + | [[Density]] is the amount of [[mass]] per [[unit]] [[Volume (Space)|volume]] of an [[object]]. | ||
| + | |||
| + | ===About Density=== | ||
| + | : The [[unit]] of [[density]] is kg/m<sup>3</sup>. | ||
| + | : An [[object]] with a large amount of [[mass]] in a small [[Volume (Space)|volume]] is said to have a high [[density]]. | ||
| + | : An [[object]] with a small amount of [[mass]] spread over a large [[Volume (Space)|volume]] is said to have a low [[density]]. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:ParticleModelSolidLiquidGas.png|center|500px]] | ||
| + | |- | ||
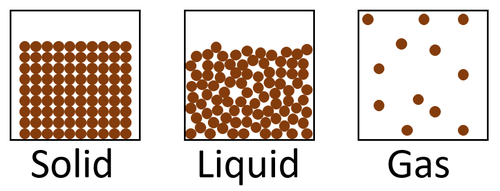
| + | | style="height:20px; width:200px; text-align:center;" |[[Solid]]s are the most '''dense''' [[State of Matter|state of matter]] because there are a large number of [[particle]]s in a certain [[Volume (Space)|volume]] and [[gas]]es are the least '''dense''' [[State of Matter|state of matter]] because there are a small number of [[particle]]s in a the same [[Volume (Space)|volume]]. | ||
| + | |} | ||
| + | |||
| + | ===Density and Floating=== | ||
| + | : If an [[object]] is more '''dense''' than [[water]] it will sink. | ||
| + | : If an [[object]] is less '''dense''' than [[water]] it will rise through [[water]] and float on the surface. | ||
| + | |||
| + | ===Equation=== | ||
| + | : Density = Mass/volume | ||
| + | |||
| + | <math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | Where: | ||
| + | : ρ = The [[density]] of the [[object]]. | ||
| + | : m = The [[mass]] of the [[object]]. | ||
| + | : V = The [[Volume (Space)|volume]] taken up by the [[object]]. | ||
| + | |||
| + | ===Example Calculations=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''5000kg of [[Iron]] has a [[Volume (Space)|volume]] of 0.635m<sup>3</sup>. Calculate the density of [[Iron]].''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''A 50,000cm<sup>3</sup> container of [[water]] is full with a 50kg [[mass]] of [[water]]. Calculate the density of [[water]].''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''A 200,000cm<sup>3</sup> [[Volume (Space)|volume]] of [[air]] has a [[mass]] of 245g. Calculate the density of [[air]]. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:left;" | | ||
| + | [[Mass]] = 5000kg | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 0.635m<sup>3</sup> | ||
| + | |||
| + | :<math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | :<math>\rho = \frac{5000}{0.635}</math> | ||
| + | |||
| + | :<math>\rho = 7874kg/m^3</math> | ||
| + | | style="height:20px; width:200px; text-align:left;" | | ||
| + | [[Mass]] = 50kg | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 50,000cm<sup>3</sup> = 0.05m<sup>3</sup> | ||
| + | |||
| + | :<math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | :<math>\rho = \frac{50}{0.05}</math> | ||
| + | |||
| + | :<math>\rho = 1000kg/m^3</math> | ||
| + | | style="height:20px; width:200px; text-align:left;" | | ||
| + | [[Mass]] = 245g = 0.245kg | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 200,000cm<sup>3</sup> = 0.2m<sup>3</sup> | ||
| + | |||
| + | :<math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | :<math>\rho = \frac{0.245}{0.2}</math> | ||
| + | |||
| + | :<math>\rho = 1.225kg/m^3</math> | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | [[Density]] is the amount of [[mass]] per [[unit]] [[Volume (Space)|volume]] of an [[object]]. | ||
| + | |||
| + | ===About Density=== | ||
| + | : The [[SI Unit]] of [[density]] is kg/m<sup>3</sup>. | ||
| + | : [[Density]] is a [[scalar]] quantity as it has [[magnitude]] but does not have a direction. | ||
| + | : An [[object]] with a large amount of [[mass]] in a small [[Volume (Space)|volume]] is said to have a high [[density]]. | ||
| + | : An [[object]] with a small amount of [[mass]] spread over a large [[Volume (Space)|volume]] is said to have a low [[density]]. | ||
| + | |||
| + | ===Finding the Density=== | ||
| + | ====Finding The Density of a Regular Object==== | ||
| + | : A regular [[object]] is a [[solid]] in the shape of a cuboid. | ||
| + | #Measure the [[mass]] of the cuboid using an [[Electronic Balance]] or [[Measuring Scale]]. | ||
| + | #Measure the length, width and height of the cuboid. | ||
| + | #Multiply the length, width and height to calculate the [[Volume (Space)|volume]]. | ||
| + | #Divide the [[mass]] by the [[Volume (Space)|volume]] of the cuboid to calculate the [[density]]. | ||
| + | |||
| + | ====Finding The Density of an Irregular Object==== | ||
| + | : An irregular [[object]] is a [[solid]] whose shape prevents the sides being measured by a [[ruler]]. | ||
| + | #Measure the [[mass]] of the [[object]] using an [[Electronic Balance]] or [[Measuring Scale]]. | ||
| + | #Fill a [[Measuring Cylinder|measuring cylinder]] with enough [[water]] to submerse the [[object]]. | ||
| + | #Take a reading of the [[Volume (Space)|volume]] of [[water]] in the [[Measuring Cylinder]]. | ||
| + | #Place the [[object]] in the [[Measuring Cylinder]] and ensure it is submersed. | ||
| + | #Take a reading of the [[Volume (Space)|volume]] of [[water]] + [[object]] in the [[Measuring Cylinder]]. | ||
| + | #Subtract the [[Volume (Space)|volume]] of [[water]] from the [[Volume (Space)|volume]] of [[water]] + [[object]] to find the [[Volume (Space)|volume]] of the [[object]]. | ||
| + | #Divide the [[mass]] by the [[Volume (Space)|volume]] of the [[object]] to calculate the [[density]]. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:ParticleModelSolidLiquidGas.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Solid]]s are the most '''dense''' [[State of Matter|state of matter]] because they have the largest amount of [[matter]] per unit [[Volume (Space)|volume]] and [[gas]]es are the least '''dense''' [[State of Matter|state of matter]] because they have the smallest amount of [[matter]] per unit [[Volume (Space)|volume]]. | ||
| + | |} | ||
| + | |||
| + | ===Density and Floating=== | ||
| + | : If an [[object]] is more '''dense''' than [[water]] it will sink. | ||
| + | : If an [[object]] is less '''dense''' than [[water]] it will rise through [[water]] and float on the surface. | ||
| + | |||
| + | ===Equation=== | ||
| + | : Density = Mass/volume | ||
| + | |||
| + | <math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | Where: | ||
| + | : ρ = The [[density]] of the [[object]]. | ||
| + | : m = The [[mass]] of the [[object]]. | ||
| + | : V = The [[Volume (Space)|volume]] taken up by the [[object]]. | ||
| + | |||
| + | ===Example Calculations=== | ||
| + | ====Finding Density from Mass and Volume==== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |5000kg of [[Iron]] has a [[Volume (Space)|volume]] of 0.635m<sup>3</sup>. Calculate the density of [[Iron]] correct to two [[Significant Figures|significant figures]].''' | ||
| + | | style="height:20px; width:300px; text-align:center;" |A 200,000cm<sup>3</sup> [[Volume (Space)|volume]] of [[air]] has a [[mass]] of 245g. Calculate the density of [[air]] correct to two [[Significant Figures|significant figures]]. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''1. State the known quantities in [[SI Unit]]s''' | ||
| + | |||
| + | [[Mass]] = 5000kg | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 0.635m<sup>3</sup> | ||
| + | |||
| + | | style="height:20px; width:300px; text-align:left;" |'''1. State the known quantities in [[SI Unit]]s''' | ||
| + | |||
| + | [[Mass]] = 245g = 0.245kg | ||
| + | |||
| + | [[Volume (Space)|Volume]] = 200,000cm<sup>3</sup> = 0.2m<sup>3</sup> | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''2. [[Substitute (Maths)|Substitute]] the numbers into the [[equation]] and [[Solve (Maths)|solve]].''' | ||
| + | |||
| + | <math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | <math>\rho = \frac{5000}{0.635}</math> | ||
| + | |||
| + | <math>\rho = 7874kg/m^3</math> | ||
| + | |||
| + | <math>\rho \approx 7900kg/m^3</math> | ||
| + | |||
| + | | style="height:20px; width:300px; text-align:left;" |'''2. [[Substitute (Maths)|Substitute]] the numbers into the [[equation]] and [[Solve (Maths)|solve]].''' | ||
| + | |||
| + | <math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | <math>\rho = \frac{0.245}{0.2}</math> | ||
| + | |||
| + | <math>\rho = 1.225kg/m^3</math> | ||
| + | |||
| + | <math>\rho \approx 1.2kg/m^3</math> | ||
| + | |} | ||
| + | |||
| + | ====Finding Volume from Mass and Density==== | ||
| + | {| class="wikitable" | ||
| + | | style="height:20px; width: 300px; text-align:center;" |[[Gold]] has a [[density]] of 19320kg/m<sup>3</sup>. 31g of [[Gold]] is used to make a coin. Calculate the [[Volume (Space)|volume]] of this coin correct to two [[Significant Figures|significant figures]]. | ||
| + | | style="height:20px; width: 300px; text-align:center;" |A 1.3ton rock with a [[density]] of 2650kg/m<sup>3</sup> is dropped into a swimming pool. Calculate the [[Volume (Space)|volume]] of [[water]] displaced by the rock, correct to two [[Significant Figures|significant figures]]. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''1. State the known quantities in [[SI Unit]]s''' | ||
| + | |||
| + | ρ = 19320kg/m<sup>3</sup> | ||
| + | |||
| + | m = 31g = 31x10<sup>-3</sup>kg | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''1. State the known quantities in [[SI Unit]]s''' | ||
| + | |||
| + | ρ = 2650kg/m<sup>3</sup> | ||
| + | |||
| + | m = 1.3ton = 1.3x10<sup>3</sup>kg | ||
| + | |- | ||
| + | | style="height:20px; width: 300px; text-align:left;" |'''2. [[Substitute (Maths)|Substitute]] the numbers and [[Evaluate (Maths)|evaluate]].''' | ||
| + | |||
| + | <math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | <math>19320 = \frac{31 \times 10^{-3}}{V}</math> | ||
| + | | style="height:20px; width: 300px; text-align:left;" |'''2. [[Substitute (Maths)|Substitute]] the numbers and [[Evaluate (Maths)|evaluate]].''' | ||
| + | |||
| + | <math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | <math>2650 = \frac{1.3 \times 10^{3}}{V}</math> | ||
| + | |- | ||
| + | | style="height:20px; width: 300px; text-align:left;" |'''3. [[Rearrange (Maths)|Rearrange]] the equation and [[Solve (Maths)|solve]].''' | ||
| + | |||
| + | <math>19320V = 31 \times 10^{-3}</math> | ||
| + | |||
| + | <math>V = \frac{31 \times 10^{-3}}{19320}</math> | ||
| + | |||
| + | <math>V = 1.60455 \times 10^{-6}m^3</math> | ||
| + | |||
| + | <math>V \approx 1.6 \times 10^{-6}</math> | ||
| + | | style="height:20px; width: 300px; text-align:left;" |'''3. [[Rearrange (Maths)|Rearrange]] the equation and [[Solve (Maths)|solve]].''' | ||
| + | |||
| + | <math>2650V = 1.3 \times 10^{3}</math> | ||
| + | |||
| + | <math>V = \frac{1.3 \times 10^{3}}{2650}</math> | ||
| + | |||
| + | <math>V = 0.490566m^3</math> | ||
| + | |||
| + | <math>V \approx 0.49m^3</math> | ||
| + | |} | ||
| + | |||
| + | ====Finding Mass from Volume and Density==== | ||
| + | {| class="wikitable" | ||
| + | | style="height:20px; width: 300px; text-align:center;" |A car is filled with 32 litres of gasoline, which has a [[density]] of 719.7kg/m<sup>3</sup>. Calculate the [[mass]] of gasoline added to the car, correct to two [[Significant Figures|significant figures]]. | ||
| + | | style="height:20px; width: 300px; text-align:center;" |A 2,500,000 litre swimming pool is filled with Chlorinated [[water]] which has a density of 993kg/m<sup>3</sup>. Calculate the [[mass]] of Chlorinated [[water]] in this swimming pool, correct to two [[Significant Figures|significant figures]]. | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''1. State the known quantities in [[SI Unit]]s''' | ||
| + | |||
| + | ρ = 719.7kg/m<sup>3</sup> | ||
| + | |||
| + | V = 32 litres = 32x10<sup>-3</sup>m<sup>3</sup> | ||
| + | | style="height:20px; width:300px; text-align:left;" |'''1. State the known quantities in [[SI Unit]]s''' | ||
| + | |||
| + | ρ = 993kg/m<sup>3</sup> | ||
| + | |||
| + | V = 2,500,000 litres = 2,500m<sup>3</sup> | ||
| + | |- | ||
| + | | style="height:20px; width: 300px; text-align:left;" |'''2. [[Substitute (Maths)|Substitute]] the numbers and [[Evaluate (Maths)|evaluate]].''' | ||
| + | |||
| + | <math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | <math>719.7 = \frac{m}{32\times10^{-3}}</math> | ||
| + | | style="height:20px; width: 300px; text-align:left;" |'''2. [[Substitute (Maths)|Substitute]] the numbers and [[Evaluate (Maths)|evaluate]].''' | ||
| + | |||
| + | <math>\rho = \frac{m}{V}</math> | ||
| + | |||
| + | <math>993 = \frac{m}{2500}</math> | ||
| + | |- | ||
| + | | style="height:20px; width: 300px; text-align:left;" |'''3. [[Rearrange (Maths)|Rearrange]] the equation and [[Solve (Maths)|solve]].''' | ||
| + | |||
| + | <math>m = 719.7 \times 32 \times 10^{-3}</math> | ||
| + | |||
| + | <math>m = 23.0304kg</math> | ||
| + | |||
| + | <math>m \approx 23kg</math> | ||
| + | | style="height:20px; width: 300px; text-align:left;" |'''3. [[Rearrange (Maths)|Rearrange]] the equation and [[Solve (Maths)|solve]].''' | ||
| + | |||
| + | <math>m = 993 \times 2500</math> | ||
| + | |||
| + | <math>m = 2482500kg</math> | ||
| + | |||
| + | <math>m \approx 2500000kg</math> | ||
| + | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09836 ''Density, page 194, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Density, page 34, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Density, page 67, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Density, pages 106-108, 170-172, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Density, pages 319, 323, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac87 ''Density, pages 38, 58, 59, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Density, pages 76-77, 164-165, 169, 204-205, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Density, pages 82, 84-7, 173, 207, 237, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Density, pages 96-98, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Density; and floating, pages 137-8, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Density; investigating, page 290, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Density; investigating, page 98, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Density; liquids, pages 68, 71, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Density; of a liquid, page 320, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Density; of a regular solid, page 321, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Density; of an irregularly shaped solid, pages 321-2, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Density; of gases, page 71, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Density; of solids, pages 69-70, 71, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Density; of water (anomaous expansion), page 91, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Density, page 183, GCSE Physics, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Density, page 415, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Density, pages 200, 201, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Density, pages 296-298, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945733/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945733&linkCode=as2&tag=nrjc-21&linkId=2a2dbec9db6bf5766c0458d908fa0a52 ''Density, pages 93, 94, 101, 102, GCSE Physics; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Density; floating, pages 321, 322, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Density; fluid pressure, pages 318, 319, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''Density; fluids, page 203, GCSE Physics, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Density; states of matter, page 300, GCSE Physics, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Density, page 151, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945687/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945687&linkCode=as2&tag=nrjc-21&linkId=9a598e52189317a20311d7a632747bc9 ''Density, pages 13, 14, Gateway GCSE Physics; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359837/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359837&linkCode=as2&tag=nrjc-21&linkId=3c4229e8b023b2b60768e7ea2307cc6f ''Density; Calculation, pages 24-25, Gateway GCSE Physics, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359837/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359837&linkCode=as2&tag=nrjc-21&linkId=3c4229e8b023b2b60768e7ea2307cc6f ''Density; Measurement, pages 250-251, Gateway GCSE Physics, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359837/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359837&linkCode=as2&tag=nrjc-21&linkId=3c4229e8b023b2b60768e7ea2307cc6f ''Density; Particle theory, pages 25, Gateway GCSE Physics, Oxford, OCR ''] | ||
Latest revision as of 14:37, 4 December 2019
Contents
Key Stage 3
Meaning
Density is the amount of mass per unit volume of an object.
About Density
- The unit of density is kg/m3.
- An object with a large amount of mass in a small volume is said to have a high density.
- An object with a small amount of mass spread over a large volume is said to have a low density.
| Solids are the most dense state of matter because there are a large number of particles in a certain volume and gases are the least dense state of matter because there are a small number of particles in a the same volume. |
Density and Floating
- If an object is more dense than water it will sink.
- If an object is less dense than water it will rise through water and float on the surface.
Equation
- Density = Mass/volume
\(\rho = \frac{m}{V}\)
Where:
Example Calculations
| 5000kg of Iron has a volume of 0.635m3. Calculate the density of Iron. | A 50,000cm3 container of water is full with a 50kg mass of water. Calculate the density of water. | A 200,000cm3 volume of air has a mass of 245g. Calculate the density of air. |
|
Mass = 5000kg Volume = 0.635m3 \[\rho = \frac{m}{V}\] \[\rho = \frac{5000}{0.635}\] \[\rho = 7874kg/m^3\] |
Mass = 50kg Volume = 50,000cm3 = 0.05m3 \[\rho = \frac{m}{V}\] \[\rho = \frac{50}{0.05}\] \[\rho = 1000kg/m^3\] |
Mass = 245g = 0.245kg Volume = 200,000cm3 = 0.2m3 \[\rho = \frac{m}{V}\] \[\rho = \frac{0.245}{0.2}\] \[\rho = 1.225kg/m^3\] |
Key Stage 4
Meaning
Density is the amount of mass per unit volume of an object.
About Density
- The SI Unit of density is kg/m3.
- Density is a scalar quantity as it has magnitude but does not have a direction.
- An object with a large amount of mass in a small volume is said to have a high density.
- An object with a small amount of mass spread over a large volume is said to have a low density.
Finding the Density
Finding The Density of a Regular Object
- Measure the mass of the cuboid using an Electronic Balance or Measuring Scale.
- Measure the length, width and height of the cuboid.
- Multiply the length, width and height to calculate the volume.
- Divide the mass by the volume of the cuboid to calculate the density.
Finding The Density of an Irregular Object
- Measure the mass of the object using an Electronic Balance or Measuring Scale.
- Fill a measuring cylinder with enough water to submerse the object.
- Take a reading of the volume of water in the Measuring Cylinder.
- Place the object in the Measuring Cylinder and ensure it is submersed.
- Take a reading of the volume of water + object in the Measuring Cylinder.
- Subtract the volume of water from the volume of water + object to find the volume of the object.
- Divide the mass by the volume of the object to calculate the density.
| Solids are the most dense state of matter because they have the largest amount of matter per unit volume and gases are the least dense state of matter because they have the smallest amount of matter per unit volume. |
Density and Floating
- If an object is more dense than water it will sink.
- If an object is less dense than water it will rise through water and float on the surface.
Equation
- Density = Mass/volume
\(\rho = \frac{m}{V}\)
Where:
Example Calculations
Finding Density from Mass and Volume
| 5000kg of Iron has a volume of 0.635m3. Calculate the density of Iron correct to two significant figures. | A 200,000cm3 volume of air has a mass of 245g. Calculate the density of air correct to two significant figures. |
| 1. State the known quantities in SI Units
Mass = 5000kg Volume = 0.635m3 |
1. State the known quantities in SI Units
Mass = 245g = 0.245kg Volume = 200,000cm3 = 0.2m3 |
| 2. Substitute the numbers into the equation and solve.
\(\rho = \frac{m}{V}\) \(\rho = \frac{5000}{0.635}\) \(\rho = 7874kg/m^3\) \(\rho \approx 7900kg/m^3\) |
2. Substitute the numbers into the equation and solve.
\(\rho = \frac{m}{V}\) \(\rho = \frac{0.245}{0.2}\) \(\rho = 1.225kg/m^3\) \(\rho \approx 1.2kg/m^3\) |
Finding Volume from Mass and Density
| Gold has a density of 19320kg/m3. 31g of Gold is used to make a coin. Calculate the volume of this coin correct to two significant figures. | A 1.3ton rock with a density of 2650kg/m3 is dropped into a swimming pool. Calculate the volume of water displaced by the rock, correct to two significant figures. |
| 1. State the known quantities in SI Units
ρ = 19320kg/m3 m = 31g = 31x10-3kg |
1. State the known quantities in SI Units
ρ = 2650kg/m3 m = 1.3ton = 1.3x103kg |
| 2. Substitute the numbers and evaluate.
\(\rho = \frac{m}{V}\) \(19320 = \frac{31 \times 10^{-3}}{V}\) |
2. Substitute the numbers and evaluate.
\(\rho = \frac{m}{V}\) \(2650 = \frac{1.3 \times 10^{3}}{V}\) |
| 3. Rearrange the equation and solve.
\(19320V = 31 \times 10^{-3}\) \(V = \frac{31 \times 10^{-3}}{19320}\) \(V = 1.60455 \times 10^{-6}m^3\) \(V \approx 1.6 \times 10^{-6}\) |
3. Rearrange the equation and solve.
\(2650V = 1.3 \times 10^{3}\) \(V = \frac{1.3 \times 10^{3}}{2650}\) \(V = 0.490566m^3\) \(V \approx 0.49m^3\) |
Finding Mass from Volume and Density
| A car is filled with 32 litres of gasoline, which has a density of 719.7kg/m3. Calculate the mass of gasoline added to the car, correct to two significant figures. | A 2,500,000 litre swimming pool is filled with Chlorinated water which has a density of 993kg/m3. Calculate the mass of Chlorinated water in this swimming pool, correct to two significant figures. |
| 1. State the known quantities in SI Units
ρ = 719.7kg/m3 V = 32 litres = 32x10-3m3 |
1. State the known quantities in SI Units
ρ = 993kg/m3 V = 2,500,000 litres = 2,500m3 |
| 2. Substitute the numbers and evaluate.
\(\rho = \frac{m}{V}\) \(719.7 = \frac{m}{32\times10^{-3}}\) |
2. Substitute the numbers and evaluate.
\(\rho = \frac{m}{V}\) \(993 = \frac{m}{2500}\) |
| 3. Rearrange the equation and solve.
\(m = 719.7 \times 32 \times 10^{-3}\) \(m = 23.0304kg\) \(m \approx 23kg\) |
3. Rearrange the equation and solve.
\(m = 993 \times 2500\) \(m = 2482500kg\) \(m \approx 2500000kg\) |
References
AQA
- Density, page 194, GCSE Combined Science; The Revision Guide, CGP, AQA
- Density, page 34, GCSE Chemistry; Student Book, Collins, AQA
- Density, page 67, GCSE Physics, Hodder, AQA
- Density, pages 106-108, 170-172, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Density, pages 319, 323, GCSE Combined Science Trilogy 1, Hodder, AQA
- Density, pages 38, 58, 59, GCSE Physics; The Revision Guide, CGP, AQA
- Density, pages 76-77, 164-165, 169, 204-205, GCSE Physics; Third Edition, Oxford University Press, AQA
- Density, pages 82, 84-7, 173, 207, 237, GCSE Physics; Student Book, Collins, AQA
- Density, pages 96-98, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Density; and floating, pages 137-8, GCSE Physics, Hodder, AQA
- Density; investigating, page 290, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Density; investigating, page 98, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Density; liquids, pages 68, 71, GCSE Physics, Hodder, AQA
- Density; of a liquid, page 320, GCSE Combined Science Trilogy 1, Hodder, AQA
- Density; of a regular solid, page 321, GCSE Combined Science Trilogy 1, Hodder, AQA
- Density; of an irregularly shaped solid, pages 321-2, GCSE Combined Science Trilogy 1, Hodder, AQA
- Density; of gases, page 71, GCSE Physics, Hodder, AQA
- Density; of solids, pages 69-70, 71, GCSE Physics, Hodder, AQA
- Density; of water (anomaous expansion), page 91, GCSE Physics; Student Book, Collins, AQA
Edexcel
- Density, page 183, GCSE Physics, Pearson Edexcel
- Density, page 415, GCSE Combined Science, Pearson Edexcel
- Density, pages 200, 201, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Density, pages 296-298, GCSE Physics, CGP, Edexcel
- Density, pages 93, 94, 101, 102, GCSE Physics; The Revision Guide, CGP, Edexcel
- Density; floating, pages 321, 322, GCSE Physics, CGP, Edexcel
- Density; fluid pressure, pages 318, 319, GCSE Physics, CGP, Edexcel
- Density; fluids, page 203, GCSE Physics, Pearson Edexcel
- Density; states of matter, page 300, GCSE Physics, CGP, Edexcel
OCR
- Density, page 151, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Density, pages 13, 14, Gateway GCSE Physics; The Revision Guide, CGP, OCR
- Density; Calculation, pages 24-25, Gateway GCSE Physics, Oxford, OCR
- Density; Measurement, pages 250-251, Gateway GCSE Physics, Oxford, OCR
- Density; Particle theory, pages 25, Gateway GCSE Physics, Oxford, OCR