Difference between revisions of "Flame Emission Spectroscopy"
| Line 8: | Line 8: | ||
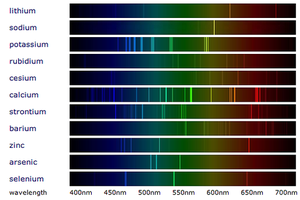
: When [[White Light|white light]] passes through a [[Spectroscope]] the colours are split into a [[spectrum]] (the rainbow). When [[Metal Compound|metal compounds]] burn they only produce certain colours so when this [[light]] is passed through a [[spectroscope]] in '''flame emission spectroscopy''' it produces very specific lines called a '[[Emission Spectra|Line Spectrum]]' instead of the broad [[spectrum]] seen from [[White Light|white light]]. | : When [[White Light|white light]] passes through a [[Spectroscope]] the colours are split into a [[spectrum]] (the rainbow). When [[Metal Compound|metal compounds]] burn they only produce certain colours so when this [[light]] is passed through a [[spectroscope]] in '''flame emission spectroscopy''' it produces very specific lines called a '[[Emission Spectra|Line Spectrum]]' instead of the broad [[spectrum]] seen from [[White Light|white light]]. | ||
: [[Metal]]s in a [[metal]] [[compound]] can be identified by comparing it to the [[Emission Spectra|line spectra]] of known [[metal]]s. | : [[Metal]]s in a [[metal]] [[compound]] can be identified by comparing it to the [[Emission Spectra|line spectra]] of known [[metal]]s. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d126 ''Flame emission spectroscopy, page 90, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Flame emission spectroscopy, pages 214-15, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Flame emission spectroscopy, pages 262, 263, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Flame emission spectroscopy, pages 263, 284-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
Latest revision as of 16:11, 15 November 2019
Key Stage 4
Meaning
Flame emission spectroscopy is a technique for identifying metals in a metal compound.
About Flame Emission Spectroscopy
- Flame emission spectroscopy is an advanced version of the Flame Tests and uses a Spectroscope to separate the colours into a spectrum.
- When white light passes through a Spectroscope the colours are split into a spectrum (the rainbow). When metal compounds burn they only produce certain colours so when this light is passed through a spectroscope in flame emission spectroscopy it produces very specific lines called a 'Line Spectrum' instead of the broad spectrum seen from white light.
- Metals in a metal compound can be identified by comparing it to the line spectra of known metals.
References
AQA
- Flame emission spectroscopy, page 90, GCSE Chemistry; The Revision Guide, CGP, AQA
- Flame emission spectroscopy, pages 214-15, GCSE Chemistry, Hodder, AQA
- Flame emission spectroscopy, pages 262, 263, GCSE Chemistry, CGP, AQA
- Flame emission spectroscopy, pages 263, 284-5, GCSE Chemistry; Student Book, Collins, AQA