Difference between revisions of "Internal Energy"
(Created page with "==Key Stage 3== ===Meaning=== Internal Energy is the total energy of all the particles in a material. ===About Internal Energy=== : The particles in a m...") |
|||
| (8 intermediate revisions by 2 users not shown) | |||
| Line 8: | Line 8: | ||
===Examples=== | ===Examples=== | ||
: [[Water]] can exist as a [[liquid]] and a [[gas]] at 100[[°C]]. The [[Kinetic Energy Store|kinetic energy]] of the [[particle]]s in both is the same. However, the [[particle]]s in [[steam]] have more '''internal energy''' than the [[particle]]s in [[water]] because they are spread apart, giving them more [[Potential Energy|potential energy]]. | : [[Water]] can exist as a [[liquid]] and a [[gas]] at 100[[°C]]. The [[Kinetic Energy Store|kinetic energy]] of the [[particle]]s in both is the same. However, the [[particle]]s in [[steam]] have more '''internal energy''' than the [[particle]]s in [[water]] because they are spread apart, giving them more [[Potential Energy|potential energy]]. | ||
| − | : The [[particle]]s in [[petrol]] at 50[[°C]] are moving around so they have [[Kinetic Energy Store|kinetic energy]]. The [[petrol]] also has a store of [[Chemical Potential Energy|chemical potential energy]] that can be released when [[petrol]] burns in the presence of [[Oxygen]]. So the '''Internal Energy''' of [[petrol]] includes both the [[Kinetic Energy Store|kinetic energy]] of the [[particle]]s and the [[Chemical Potential Energy|chemical potential energy]]. | + | : The [[particle]]s in [[petrol]] at 50[[°C]] are moving around so they have [[Kinetic Energy Store|kinetic energy]]. The [[petrol]] also has a store of [[Chemical Potential Energy Store|chemical potential energy]] that can be released when [[petrol]] burns in the presence of [[Oxygen]]. So the '''Internal Energy''' of [[petrol]] includes both the [[Kinetic Energy Store|kinetic energy]] of the [[particle]]s and the [[Chemical Potential Energy Store|chemical potential energy]]. |
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | [[Internal Energy]] is the sum of all [[Kinetic Energy Store|kinetic]] and [[Potential Energy|potential energies]] of [[particle]]s in a [[system]]. | ||
| + | |||
| + | ===About Internal Energy=== | ||
| + | : The [[particle]]s in a [[material]] have [[Kinetic Energy Store|kinetic energy]], because they move, and several types of [[Potential Energy|potential energy]] because there are [[force]]s between [[adjacent]] [[particle]]s. | ||
| + | : [[Force]]s of [[attraction]], including [[Chemical Bond|chemical bonds]], pull [[particle]]s together. The further apart the [[particle]]s the more [[Potential Energy|potential energy]] they poses. As a result the [[gas]]eous [[State of Matter|state]] of a [[substance]] has more [[Potential Energy|potential energy]] than the [[liquid]] [[State of Matter|state]] of that [[substance]], even if the [[temperature]] of both is the same because the [[particle]]s are spread apart in a [[gas]] and close together in a [[liquid]]. | ||
| + | : Increasing the '''internal energy''' of a [[substance]] by [[heating]] does not always increase its [[temperature]]. When a [[substance]] [[State Change|changes state]] the '''internal energy''' changes but the [[Thermal Energy Store|thermal energy]] does not, so it remains at a constant [[temperature]]. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |[[File:MeltingGraph.png|center|300px]] | ||
| + | |[[File:BoilingGraph.png|center|300px]] | ||
| + | |- | ||
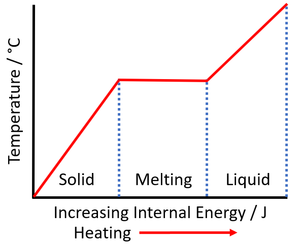
| + | | style="height:20px; width:300px; text-align:left;" |As a [[solid]] is [[heated]] the [[Internal Energy|internal energy]] increases. As the [[solid]] '''melts''' the [[temperature]] of the [[substance]] stays the same but the [[Potential Energy|potential energy]] of the [[particle]]s continues to increase. | ||
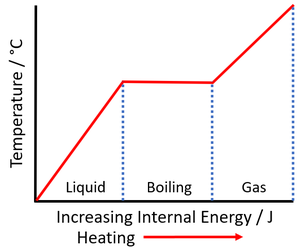
| + | | style="height:20px; width:300px; text-align:left;" |As a [[liquid]] is [[heated]] the [[Internal Energy|internal energy]] increases. As the [[liquid]] '''boils''' the [[temperature]] of the [[substance]] stays the same but the [[Potential Energy|potential energy]] of the [[particle]]s continues to increase. | ||
| + | |} | ||
| + | ===Equation=== | ||
| + | |||
| + | '' '''Internal Energy''' = All [[Kinetic Energy Store|kinetic energies]] + All [[Potential Energy|potential energies]]'' | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Internal (thermal) energy, pages 2-3, 27, 72, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Internal (thermal) energy, pages 259, 260, 282, 324, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851370/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851370&linkCode=as2&tag=nrjc-21&linkId=01c69b0ae058f809cf636033e6ba793e ''Internal (thermal) energy; and specific heat capacity, pages 12-14, GCSE Physics, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Internal (thermal) energy; changes in, page 326, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Internal energy stores, page 21, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Internal energy stores, page 22, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''Internal energy, pages 100-104, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Internal energy, pages 110-115, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe10006 ''Internal energy, pages 195, 196, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac257 ''Internal energy, pages 39-41, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Internal energy, pages 82-83, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Internal energy, pages 83, 90-1, 97, 100-1, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''Internal energy, page 301, GCSE Physics, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945687/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945687&linkCode=as2&tag=nrjc-21&linkId=9a598e52189317a20311d7a632747bc9 ''Internal energy, page 15, Gateway GCSE Physics; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Internal energy, page 153, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359837/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359837&linkCode=as2&tag=nrjc-21&linkId=3c4229e8b023b2b60768e7ea2307cc6f ''Internal energy, pages 28, Gateway GCSE Physics, Oxford, OCR ''] | ||
Latest revision as of 15:30, 13 December 2019
Contents
Key Stage 3
Meaning
Internal Energy is the total energy of all the particles in a material.
About Internal Energy
- The particles in a material have kinetic energy because they move and several types of potential energy because they are affected by forces. Internal Energy is all of those energies added together.
Examples
- Water can exist as a liquid and a gas at 100°C. The kinetic energy of the particles in both is the same. However, the particles in steam have more internal energy than the particles in water because they are spread apart, giving them more potential energy.
- The particles in petrol at 50°C are moving around so they have kinetic energy. The petrol also has a store of chemical potential energy that can be released when petrol burns in the presence of Oxygen. So the Internal Energy of petrol includes both the kinetic energy of the particles and the chemical potential energy.
Key Stage 4
Meaning
Internal Energy is the sum of all kinetic and potential energies of particles in a system.
About Internal Energy
- The particles in a material have kinetic energy, because they move, and several types of potential energy because there are forces between adjacent particles.
- Forces of attraction, including chemical bonds, pull particles together. The further apart the particles the more potential energy they poses. As a result the gaseous state of a substance has more potential energy than the liquid state of that substance, even if the temperature of both is the same because the particles are spread apart in a gas and close together in a liquid.
- Increasing the internal energy of a substance by heating does not always increase its temperature. When a substance changes state the internal energy changes but the thermal energy does not, so it remains at a constant temperature.
| As a solid is heated the internal energy increases. As the solid melts the temperature of the substance stays the same but the potential energy of the particles continues to increase. | As a liquid is heated the internal energy increases. As the liquid boils the temperature of the substance stays the same but the potential energy of the particles continues to increase. |
Equation
Internal Energy = All kinetic energies + All potential energies
References
AQA
- Internal (thermal) energy, pages 2-3, 27, 72, GCSE Physics, Hodder, AQA
- Internal (thermal) energy, pages 259, 260, 282, 324, GCSE Combined Science Trilogy 1, Hodder, AQA
- Internal (thermal) energy; and specific heat capacity, pages 12-14, GCSE Physics, Hodder, AQA
- Internal (thermal) energy; changes in, page 326, GCSE Combined Science Trilogy 1, Hodder, AQA
- Internal energy stores, page 21, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Internal energy stores, page 22, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Internal energy, pages 100-104, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Internal energy, pages 110-115, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Internal energy, pages 195, 196, GCSE Combined Science; The Revision Guide, CGP, AQA
- Internal energy, pages 39-41, GCSE Physics; The Revision Guide, CGP, AQA
- Internal energy, pages 82-83, GCSE Physics; Third Edition, Oxford University Press, AQA
- Internal energy, pages 83, 90-1, 97, 100-1, GCSE Physics; Student Book, Collins, AQA