Difference between revisions of "Periodic Table"
| Line 11: | Line 11: | ||
|- | |- | ||
|[[File:PeriodicTable.png|center|900px]] | |[[File:PeriodicTable.png|center|900px]] | ||
| + | |} | ||
| + | |||
| + | ===Groups=== | ||
| + | : The [[element]]s are arranged [[group]]s of similar [[Chemical Properties]]. | ||
| + | : [[Element]]s have similar [[Chemical Properties]] when they have the same number of [[electron]]s in the [[Outer Shell]]. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:PeriodicTableGroups.png|center|600px]] | ||
| + | |} | ||
| + | |||
| + | ===Periods=== | ||
| + | : The [[period]]s are arranged by the number of [[Electron Shell]]s. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:PeriodicTablePeriods.png|center|600px]] | ||
|} | |} | ||
Revision as of 10:07, 30 September 2018
Key Stage 3
Meaning
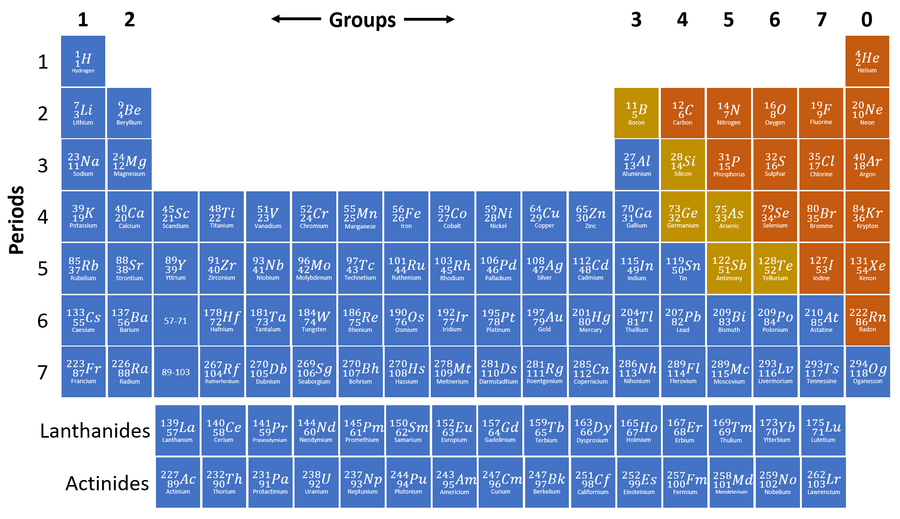
The Periodic Table is a chart listing all the known elements arranged in order of Atomic Number and in columns of elements with similar properties.
About The Periodic Table
- The modern Periodic Table was arranged by a scientist called Mendeleev. Other's had tried to arrange all the elements before, but Mendeleev was the first to arrange by both Atomic Number and chemical properties.
- The columns of the Periodic Table are called Groups.
- The rows of the Periodic Table are called Periods.
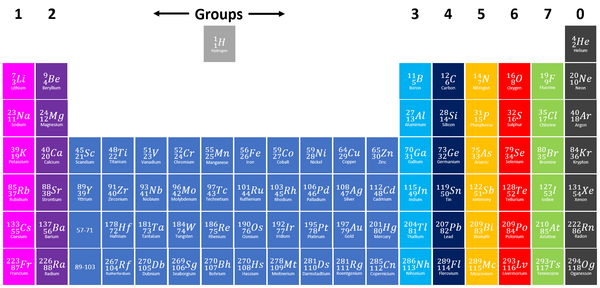
Groups
- The elements are arranged groups of similar Chemical Properties.
- Elements have similar Chemical Properties when they have the same number of electrons in the Outer Shell.
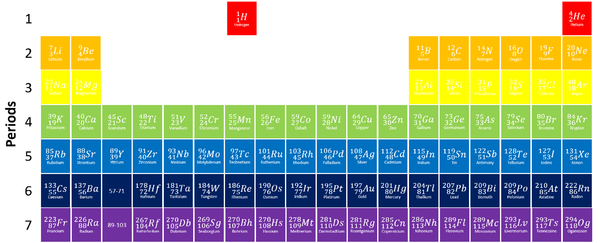
Periods
- The periods are arranged by the number of Electron Shells.