Difference between revisions of "Solution"
(→Separating Solutions) |
(→Separating Solutions) |
||
| Line 58: | Line 58: | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| − | |[[File:Distillation.png|center| | + | |[[File:Distillation.png|center|600px]] |
|- | |- | ||
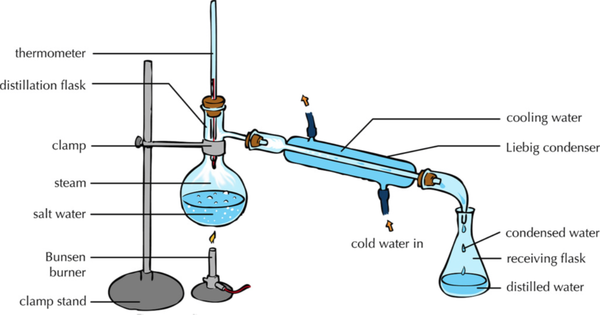
| − | | style="height:20px; width: | + | | style="height:20px; width:600px; text-align:center;" |This [[diagram]] shows how the [[solute]] and [[solvent]] can be [[Separating Mixtures|separated]] by heating the [[solution]] to [[evaporate]] the [[solvent]] leaving the [[solute]] behind and then [[condensing]] the [[evaporate]]d [[solvent]] by cooling it down and collecting it in a [[Conical Flask|conical flask]]. |
|} | |} | ||
Revision as of 10:18, 27 September 2018
Contents
Key Stage 2
Meaning
A solution is a mixture where a substance is dissolved in a liquid.
About Solutions
- For most solutions the liquid is water.
- Some solids dissolve in water to make a solution.
- Once a substance is dissolved in water it is often not possible to see it anymore.
- If a substance has a colour before it is dissolved the solution be will that colour.
Examples
| Salt | Sugar |
| Salt dissolves in water to make a solution called Brine. | Sugar dissolves in water to make a solution. |
Key Stage 3
Meaning
About Solutions
- Solutions are usually made of liquid that is known as a solvent and a solid which is known as a solute.
- When a solute dissolves in a solvent it becomes a solution.
- Some solutions are a mixture of two solvents, for example water and ethanol.
Examples
| Brown sugar is the solute. | Water is the solvent. | The solute dissolves in the solvent to make a solution. |
| Copper Sulphate is the solute. | Water is the solvent. | The solute dissolves in the solvent to make a solution. |
Separating Solutions
- The solute and solvent can be separated by distillation.
| This diagram shows how the solute and solvent can be separated by heating the solution to evaporate the solvent leaving the solute behind and then condensing the evaporated solvent by cooling it down and collecting it in a conical flask. |