Diamond
Contents
Key Stage 3
Meaning
Diamonds are large crystal molecules of carbon.
About Diamonds
- Diamonds are very hard.
- Diamonds are transparent.
Key Stage 4
Meaning
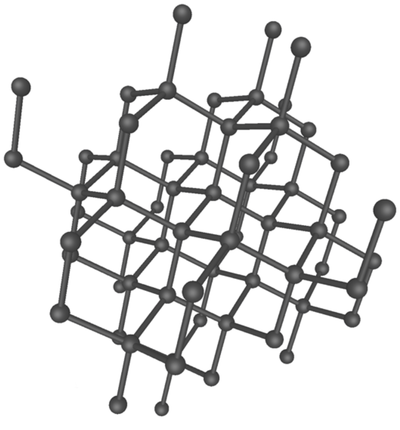
Diamond is an allotrope of carbon in which the atoms are bonded in a tetrahedral arrangement.
| A diagram showing the arrangement of carbon atoms in diamond. |
About Diamonds
- Diamonds are a giant covalent structure.
- Diamonds are extremely hard.
- Diamonds have a high melting point.
- Diamonds are transparent and have a high refractive index.
- Diamond is a poor electrical conductor because there are no free electrons to move around the giant covalent structure.
References
AQA
- Diamond, page 166, GCSE Combined Science Trilogy 1, Hodder, AQA
- Diamond, page 54, GCSE Chemistry, Hodder, AQA
- Diamond, pages 118, 119, GCSE Combined Science; The Revision Guide, CGP, AQA
- Diamond, pages 33, 34, GCSE Chemistry; The Revision Guide, CGP, AQA
- Diamond, pages 48-49, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Diamond, pages 56-7, 64, 76-7, 80-1, 91, GCSE Chemistry; Student Book, Collins, AQA
- Diamond, pages 86, 87, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Diamond, pages 88, 89, GCSE Chemistry, CGP, AQA
Edexcel
- Diamond, page 24, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Diamond, page 62, GCSE Chemistry, CGP, Edexcel