Electron Capture
Key Stage 5
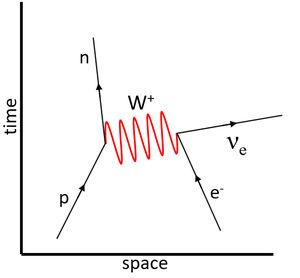
This is a Feynman diagram showing Electron capture due to the weak interaction in which a proton captures an electron to become a neutron.
Meaning
Electron capture is a process in which a proton-rich nucleus absorbs an inner-shell electron, causing a proton to transmute into a neutron and emitting a neutrino.
About Electron Capture
- Electron capture typically occurs in proton-rich isotopes.
- The captured electron combines with a proton to form a neutron and an electron neutrino (νe).
- Electron capture reduces the atomic number by one while the mass number remains the same.
- Electron capture is often followed by the emission of X-rays as electrons from higher energy levels fall into the lower energy vacancy.
- Electron capture helps to achieve a more stable nucleus by reducing proton number.
Examples
- Electron capture occurs in Beryllium-7 to form Lithium-7.
- During the the weak interaction by which electron capture occurs several quantities can be shown to be conserved:
| \(p\) | \(+\) | \(e^-\) | \(\rightarrow\) | \(n\) | \(+\) | \(\nu_e\) | |
| Charge | \(+1\) | \(+\) | \(-1\) | \(=\) | \(0\) | \(+\) | \(0\) |
| Baryon Number | \(+1\) | \(+\) | \(0\) | \(=\) | \(+1\) | \(+\) | \(0\) |
| Lepton Number | \(0\) | \(+\) | \(+1\) | \(=\) | \(0\) | \(+\) | \(+1\) |
| Strangeness | \(0\) | \(+\) | \(0\) | \(=\) | \(0\) | \(+\) | \(0\) |