Evaporation of Solutions
Contents
Key Stage 2
Meaning
Evaporation of a solution is a way to get back a solid that has been dissolved in a liquid.
About Evaporation of Solutions
| You can separate salt from the water by evaporating the water in an evaporating dish. |
Examples
| These people collect salt by putting sea water in small ponds and allowing the warm temperatures to evaporate the water away leaving the behind the salt. |
Key Stage 3
Meaning
Evaporation of a solution is a way to separate the mixture of a solution to recover the solute that has been dissolved in a solvent.
About Evaporation of Solutions
- The evaporation of solutions recovers the solutes but loses the solvent.
- Evaporation of solutions can be done by directly heating the solution or by giving time for the liquid to evaporate at low temperatures.
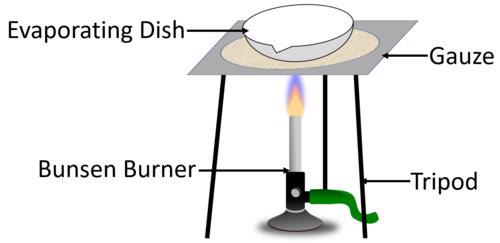
| The diagram shows the experimental setup to evaporate away the solvent to leave behind the solute in the evaporating dish. |
Key Stage 4
Meaning
Evaporation of solution, sometimes called Crystallisation, is a technique used to separate the solute from the solvent in a solution, losing the solvent.
About Evaporation of Solutions
Evaporation of solutions can only be used for:
- Separating a solution to recover solutes from the solutions.
- Separating insoluble solids from a liquid. However, this is unnecessary as it can be done by filtration.
Evaporation of solutions cannot be used for:
- Recovering the solvent from a solution - Distillation
- Separating two solutes from each other in solution - Chromatography
- Separating an insoluble solid from a soluble solid - Filtration
- Separating two solvents from each other in solution - Fractional Distillation