Plum Pudding Model
Contents
Key Stage 4
Meaning
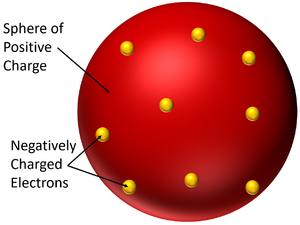
The Plum Pudding Model is a model of the atom which suggests the atom is a solid sphere of positive charge with negatively charged electrons spread within it.
About the Plum Pudding Model
- In the Plum Pudding Model the atom is neutral because the negatively charged electrons are fixed within a larger sphere of positive charge.
- The Plum Pudding Model is named after a desert made from sponge with plums stuck inside. It was imagined that the atom had a sphere of positive charge like the sponge of the cake and electrons suck inside, like the plums stuck inside the sponge.
- The Plum Pudding Model was proposed by J.J. Thompson who discovered the electron and realised it was part of an atom.
- The Plum Pudding Model was proven false by Rutherford's Alpha Scattering Experiment and was replaced by the Nuclear Model.
References
AQA
- Plum pudding model, page 42, GCSE Chemistry, CGP, AQA
- Plum pudding model, page 104, GCSE Combined Science; The Revision Guide, CGP, AQA
- Plum pudding model, page 108, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Plum pudding model, page 120, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Plum pudding model, page 132, GCSE Physics; Student Book, Collins, AQA
- Plum pudding model, page 19, GCSE Chemistry; The Revision Guide, CGP, AQA
- Plum pudding model, page 42, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Plum pudding model, page 43, GCSE Physics; The Revision Guide, CGP, AQA
- Plum pudding model, pages 94-95, GCSE Physics; Third Edition, Oxford University Press, AQA
- Plum-pudding model of the atom, pages 85, 91, GCSE Physics, Hodder, AQA
Edexcel
- Plum pudding model, page 149, GCSE Physics, CGP, Edexcel
- Plum pudding model, page 15, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Plum pudding model, page 32, GCSE Chemistry, CGP, Edexcel
- Plum pudding model, pages 78, 172, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Plum-pudding model, page 49, GCSE Physics; The Revision Guide, CGP, Edexcel