Rutherford's Alpha Scattering Experiment
Contents
Key Stage 4
Meaning
Rutherford's Alpha Scattering Experiment was an experiment that provided evidence which disproved the Plum Pudding Model of the atom and later led to the development of the Nuclear Model of the atom.
About Rutherford's Alpha Scattering Experiment
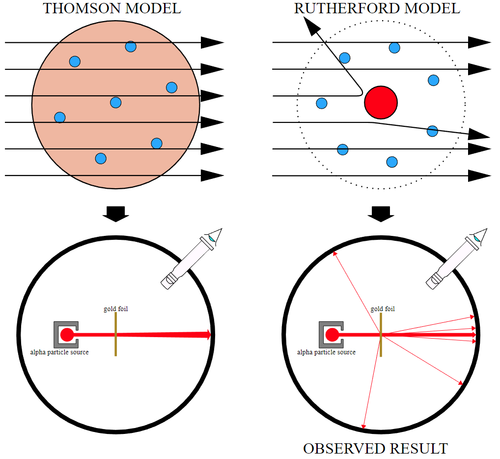
- In the experiment Rutherford's students (Ernest Marsden and Hans Geiger) fired high speed alpha particles at a thin sheet of gold foil. A detector could be placed at different positions around the experiment which would make a small flash of light every time an alpha particle reached the detector. This could be used to find the path of the alpha particles as they passed through the gold foil.
- The path of the alpha particles provided evidence to disprove the Plum Pudding Model.
- If the Plum Pudding Model were correct then nearly all of the alpha particles should have passed straight through, unaffected, since the alpha particle is positively charged while atoms should have an even spread of charged particles all the way through them. There should have been no electrostatic force to change their direction.
- They observed that most of the alpha particles went through in a straight line. A small number were deflected by a small angle and a very small number bounced off the Gold back towards the alpha source.
These observations led to a number of conclusions:
| Observation | Conclusion |
| Most of the alpha particles pass straight through the foil. | The atom must be mostly empty space. |
| Some of the alpha particles were deflected by a small angle. | The mass of the atom must be concentrated in an extremely small volume in the centre. |
| A very small number of alpha particles came back in the direction of the detector. (Deflected more than 90°.) | The centre of an atom must have a strong positive charge.
The electrons must not be in the centre of the atom, they must be orbiting the nucleus. |
| This diagram shows the expected path of alpha particles through an atom in the Plum Pudding Model (Thompson Model) and what was actually observed, which led to the development of the Nuclear Model (Rutherford Model). |