Subliming
Contents
Key Stage 3
Meaning
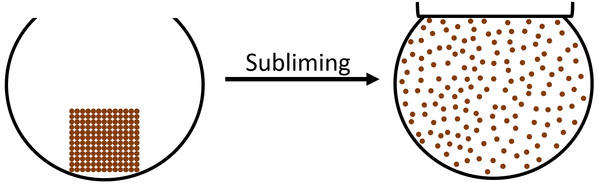
Subliming is an endothermic process in which a solid turns directly into a gas without going through a liquid state.
- Noun: Sublimation
- Verb: To sublime
- Present Participle: Subliming
About Subliming
- Only a few solids sublime. Frozen Carbon Dioxide will sublime which means it skips the liquid state.
- Sublimation is a reversible process. When a solid sublimes into a gas you can deposit that gas back into a solid.
- Heating a solid will cause it to sublime into a gas.
| The particles in the solid vibrate faster until they are moving fast enough that they break the bonds holding the particles together. The particles become free to move anywhere which makes the state a gas. |
Key Stage 4
Meaning
Subliming is an endothermic physical change in which a solid turns into a gas without going through a liquid phase.
About Subliming
- Subliming happens when the particles in a solid break bonds holding them together in fixed positions as they gain potential energy.
- Subliming is an endothermic process, which means it needs to absorb energy to take place.
- Subliming is a physical change, which means it is reversible and does not produce new chemicals.
- Substances may sublime depending on the pressure around them. In atmospheric pressure (101,000Pa) Carbon Dioxide and Iodine will both sublime and cannot exist in a liquid state. However, under extremely high pressure Carbon Dioxide can be turned into a liquid.
- Ice will sublime in a vacuum but not in normal atmospheric pressure.
References
AQA
- Sublimation, page 195, GCSE Combined Science; The Revision Guide, CGP, AQA
- Sublimation, page 324, GCSE Combined Science Trilogy 1, Hodder, AQA
- Sublimation, page 39, GCSE Physics; The Revision Guide, CGP, AQA
- Sublimation, page 68, GCSE Chemistry; Student Book, Collins, AQA
- Sublimation, page 72, GCSE Physics, Hodder, AQA