Difference between revisions of "Methanoic Acid"
| Line 34: | Line 34: | ||
: <chem>2HCOOH + 2K -> 2HCOOK + H2O</chem> | : <chem>2HCOOH + 2K -> 2HCOOK + H2O</chem> | ||
| − | : [[Methanoic Acid]] + [[Lithium Hydroxide]] → [[ | + | : [[Methanoic Acid]] + [[Lithium Hydroxide]] → [[Lithium Methanoate]] + [[Water]] |
: <chem>HCOOH + LiOH -> HCOOLi + H2O</chem> | : <chem>HCOOH + LiOH -> HCOOLi + H2O</chem> | ||
: [[Methanoic Acid]] + [[Magnesium Carbonate]] → [[Magnesium Methanoate]] + [[Water]] | : [[Methanoic Acid]] + [[Magnesium Carbonate]] → [[Magnesium Methanoate]] + [[Water]] | ||
: <chem>2HCOOH + MgCO3 -> (HCOO)2Mg + CO2 + H2O</chem> | : <chem>2HCOOH + MgCO3 -> (HCOO)2Mg + CO2 + H2O</chem> | ||
Revision as of 11:48, 3 April 2019
Contents
Key Stage 3
Meaning
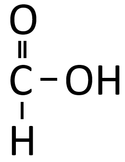
Methanoic Acid is a compound with chemical formula CH2O2.
About Methanoic Acid
- Methanoic Acid is a solid dissolved in water to form a solution.
- Methanoic Acid can be oxidised to produce Carbon Dioxide and Water.
- Methanoic Acid + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Methanoic Acid is a Carboxylic Acid with chemical formula CH2O2.
About Methanoic Acid
- Methanoic Acid is an aqueous solution.
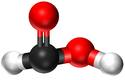
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| CH2O2 | HCOOH |
Methanoic Acid can be neutralised to produce organic salts.
- Methanoic Acid + Sodium → Sodium Methanoate + Hydrogen
- <chem>2HCOOH + 2Na -> 2HCOONa + H2</chem>
- Methanoic Acid + Potassium Oxide → Potassium Methanoate + Water
- <chem>2HCOOH + 2K -> 2HCOOK + H2O</chem>
- Methanoic Acid + Lithium Hydroxide → Lithium Methanoate + Water
- <chem>HCOOH + LiOH -> HCOOLi + H2O</chem>
- Methanoic Acid + Magnesium Carbonate → Magnesium Methanoate + Water
- <chem>2HCOOH + MgCO3 -> (HCOO)2Mg + CO2 + H2O</chem>