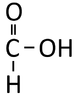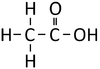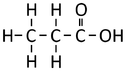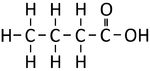Difference between revisions of "Carboxylic Acid"
(→Esterification) |
|||
| Line 60: | Line 60: | ||
[[Carboxylic Acid]]s may [[Chemical Reaction|react]] with [[alcohol]]s to [[product|produce]] [[compound]]s known as [[ester]]s which have the [[Functional Group|functional group]] -COO-. | [[Carboxylic Acid]]s may [[Chemical Reaction|react]] with [[alcohol]]s to [[product|produce]] [[compound]]s known as [[ester]]s which have the [[Functional Group|functional group]] -COO-. | ||
: [[Methanoic Acid]] + [[Ethanol]] → Ethyl Methanoate + [[Water]] | : [[Methanoic Acid]] + [[Ethanol]] → Ethyl Methanoate + [[Water]] | ||
| − | + | <math>HCOOH + C2_H_5OH -> HCOOC_2H_5 + H_2O</math> | |
: [[Ethanoic Acid]] + [[Ethanol]] → Ethyl Ethanoate + [[Water]] | : [[Ethanoic Acid]] + [[Ethanol]] → Ethyl Ethanoate + [[Water]] | ||
| − | + | <math>CH_3COOH + C_2H_5OH -> CH_3COOC_2H_5 + H_2O</math> | |
: [[Propanoic Acid]] + [[Propanol]] → Propyl Propanoate + [[Water]] | : [[Propanoic Acid]] + [[Propanol]] → Propyl Propanoate + [[Water]] | ||
| − | + | <math>C_3H_5COOH + C_3H_7OH -> C_2H_5COOC_3H_7 + H_2O</math> | |
: [[Butanoic Acid]] + [[Propanol]] → Propyl Butanoate + [[Water]] | : [[Butanoic Acid]] + [[Propanol]] → Propyl Butanoate + [[Water]] | ||
| − | + | <math>C_3H_5COOH + C_3H_7OH -> C_3H_5COOC_3H_7 + H_2O</math> | |
Revision as of 13:13, 7 June 2019
Contents
Key Stage 4
Meaning
Carboxylic Acids are organic compounds with a Carbon atom which has a double bonds to an Oxygen atom and a single bond to an OH group. The general formula is CnH2nO2.
About Carboxylic Acids
- Carboxylic Acids are a homologous series of organic compounds.
- The functional group of the Carboxylic Acids is the double bond between a Carbon atom and an Oxygen atom and the single bond to an OH group.
- Carboxylic Acids are long chains of Carbon atoms covalently bonded together with the the final Carbon atom in the chain as a COOH group.
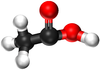
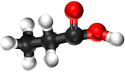
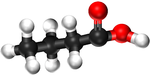
Examples
| Methanoic Acid | Ethanoic Acid | Propanoic Acid | Butanoic Acid | |
| Chemical Formula | CH2O2 | C2H4O2 | C3H6O2 | C4H8O2 |
| Structural Formula | HCOOH | CH3COOH | CH3CH2COOH | CH3CH2CH2COOH |
| Structural Diagram | ||||
| Ball and Stick Model |
Reactions of Carboxylic Acids
Neutralisation
Carboxylic Acids can be neutralised to produce organic salts.
\(2HCOOH + 2K → 2HCOOK + H_2\)
\(2CH_3COOH + MgO → (CH3COO)_2Mg + H_2O\)
\(C_2H_5COOH + NaOH → C_2H_5COONa + H_2O\)
\(2C_3H_7COOH + MgCO_3 → (C_3H_7COO)_2Mg + H_2O + CO_2\)
Esterification
Carboxylic Acids may react with alcohols to produce compounds known as esters which have the functional group -COO-.
- Methanoic Acid + Ethanol → Ethyl Methanoate + Water
\(HCOOH + C2_H_5OH -> HCOOC_2H_5 + H_2O\)
- Ethanoic Acid + Ethanol → Ethyl Ethanoate + Water
\(CH_3COOH + C_2H_5OH -> CH_3COOC_2H_5 + H_2O\)
- Propanoic Acid + Propanol → Propyl Propanoate + Water
\(C_3H_5COOH + C_3H_7OH -> C_2H_5COOC_3H_7 + H_2O\)
- Butanoic Acid + Propanol → Propyl Butanoate + Water
\(C_3H_5COOH + C_3H_7OH -> C_3H_5COOC_3H_7 + H_2O\)