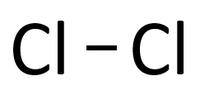Single Bond
Key Stage 4
Meaning
A single bond is a chemical bond in which only one electron is shared or transferred from the outer shell.
About Single Bonds
- In covalent bonds a single bond means one electron from the outer shell of an atom is shared.
- In ionic bonds a single bond means one of the atoms has gained or lost just one electron that has been transferred to another element.
Examples
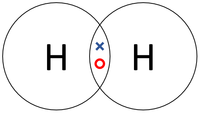
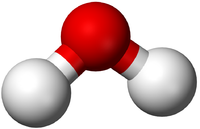
| In this structural diagram a single bond is shown between two Chlorine atoms. | In this dot and cross diagram the two Hydrogen atoms in a Hydrogen molecule are shown to each share only one electron in a single bond. | In this ball and stick model of Water the Oxygen in Water shares one electron (shown by one stick) with each Hydrogen atom forming two single bonds. |