Difference between revisions of "Conservation of Mass"
(→Calculating the Mass of a missing Product/Reactant) |
|||
| (14 intermediate revisions by 2 users not shown) | |||
| Line 36: | Line 36: | ||
===About Conservation of Mass=== | ===About Conservation of Mass=== | ||
: In a [[Chemical Reaction|chemical reaction]] law of '''conservation of mass''' indicates that the total [[mass]] of the [[product]]s is the same as the total [[mass]] of the [[reactant]]s. | : In a [[Chemical Reaction|chemical reaction]] law of '''conservation of mass''' indicates that the total [[mass]] of the [[product]]s is the same as the total [[mass]] of the [[reactant]]s. | ||
| + | : '''Conservation of mass''' can be [[observed]] in [[Closed System|closed system]]s in which none of the [[product]]s can escape and no other [[chemical]]s enter the [[system]]. | ||
| + | : In an [[Open System|open system]] any [[Chemical Reaction|chemical reaction]] which [[product|produces]] a [[gas]] will appear to decrease in [[mass]] but only because the [[mass]] has moved to a different location. The [[particle]]s of [[gas]] escape the container. | ||
===Examples=== | ===Examples=== | ||
| Line 98: | Line 100: | ||
{| class="wikitable" | {| class="wikitable" | ||
| style="height:20px; width:200px; text-align:center;" | | | style="height:20px; width:200px; text-align:center;" | | ||
| − | Find the [[mass]] of [[ | + | Find the [[mass]] of [[Oxygen]] needed to completely [[oxidise]] all of the [[Magnesium]]: |
| − | + | 2Mg + O<sub>2</sub> → 2MgO | |
| − | + | 48g + x = y | |
| style="height:20px; width:200px; text-align:center;" | | | style="height:20px; width:200px; text-align:center;" | | ||
| − | Find the [[mass]] of [[ | + | Find the [[mass]] of [[Oxygen]] needed for the [[Complete Combustion|complete combustion]] of [[Methane]]. |
CH<sub>4</sub> + 2O<sub>2</sub> → 2H<sub>2</sub>O + CO<sub>2</sub> | CH<sub>4</sub> + 2O<sub>2</sub> → 2H<sub>2</sub>O + CO<sub>2</sub> | ||
| − | + | 32g + x = y | |
| style="height:20px; width:200px; text-align:center;" | | | style="height:20px; width:200px; text-align:center;" | | ||
| − | Find the [[mass]] of [[Hydrochloric Acid]] needed | + | Find the [[mass]] of [[Hydrochloric Acid]] needed to completely [[Neutralise (Chemistry)|neutralise]] all of the [[Sodium Hydroxide]]. |
NaOH + HCl → NaCl + H<sub>2</sub>O | NaOH + HCl → NaCl + H<sub>2</sub>O | ||
| − | + | 20g + x = y + z | |
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | Find the [[Relative Formula Mass]] of the [[reactant]]s. | ||
| + | |||
| + | M<sub>r</sub> of Mg = 24g | ||
| + | |||
| + | M<sub>r</sub> of O<sub>2</sub> = 16x2 | ||
| + | |||
| + | M<sub>r</sub> of O<sub>2</sub> = 32g | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | Find the [[Relative Formula Mass]] of the [[reactant]]s. | ||
| + | |||
| + | M<sub>r</sub> of CH<sub>4</sub> = 16g | ||
| + | |||
| + | M<sub>r</sub> of O<sub>2</sub> = 16x2 | ||
| + | |||
| + | M<sub>r</sub> of O<sub>2</sub> = 32g | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | Find the [[Relative Formula Mass]] of the [[reactant]]s. | ||
| + | |||
| + | M<sub>r</sub> of NaOH = 40g | ||
| + | |||
| + | M<sub>r</sub> of HCl = 36.5g | ||
| + | |||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | State the [[ratio]] of [[mole]]s of each [[chemical]] needed. | ||
| + | |||
| + | 2 [[mole]]s of Mg are needed for every 1 [[mole]] of O<sub>2</sub> | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | State the [[ratio]] of [[mole]]s of each [[chemical]] needed. | ||
| + | |||
| + | 1 [[mole]] of CH<sub>4</sub> is needed for every 2 [[mole]]s of O<sub>2</sub> | ||
| + | |||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | State the [[ratio]] of [[mole]]s of each [[chemical]] needed. | ||
| + | |||
| + | 1 [[mole]] of HCl are needed for every 1 [[mole]] of NaOH | ||
| + | |||
|- | |- | ||
| + | |||
| style="height:20px; width:200px; text-align:center;" | | | style="height:20px; width:200px; text-align:center;" | | ||
| − | + | Find the number of [[mole]]s supplied of the known [[mass]]. | |
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{48}{24}</math> | ||
| + | |||
| + | No. [[Mole]]s = 2 Mole | ||
| + | |||
| + | Therefore 1 [[mole]] of O<sub>2</sub> is needed. | ||
| + | |||
| + | 1 [[mole]] of O<sub>2</sub> = 32g | ||
| − | |||
| style="height:20px; width:200px; text-align:center;" | | | style="height:20px; width:200px; text-align:center;" | | ||
| − | + | Find the number of [[mole]]s supplied of the known [[mass]]. | |
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{32}{16}</math> | ||
| + | |||
| + | No. [[Mole]]s = 2 Mole | ||
| − | + | Therefore 4 [[mole]]s of O<sub>2</sub> are needed. | |
| − | + | 4 [[mole]]s of O<sub>2</sub> = 128g | |
| style="height:20px; width:200px; text-align:center;" | | | style="height:20px; width:200px; text-align:center;" | | ||
| − | + | Find the number of [[mole]]s supplied of the known [[mass]]. | |
| + | |||
| + | No. [[Mole]]s = <math>\frac{Mass}{M_r}</math> | ||
| + | |||
| + | No. [[Mole]]s = <math>\frac{20}{40}</math> | ||
| + | |||
| + | No. [[Mole]]s = 0.5 Mole | ||
| + | |||
| + | Therefore 0.5 [[mole]] of HCl is needed. | ||
| − | + | 0.5 [[mole]] of HCl = 18.25g | |
| − | |||
|} | |} | ||
| + | |||
| + | ==Beyond the Curriculum== | ||
| + | {{#ev:youtube|https://www.youtube.com/watch?v=2S6e11NBwiw}} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Conservation of mass, page 181, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d91 ''Conservation of mass, page 43, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Conservation of mass, page 6, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Conservation of mass, page 68, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Conservation of mass, page 78, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Conservation of mass, pages 108-110, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Conservation of mass, pages 114- 116, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09801 ''Conservation of mass, pages 124, 125, 195, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Conservation; of mass, pages 88, 116-17, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Conservation of mass, apge 26, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Conservation of mass, law of, pages 218-219, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Conservation of mass, law of, pages 74-75, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Conservation of mass, page 89, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Conservation of mass, pages 74-76, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Mass conservation, pages 74-75, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Conservation of mass, page 107, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Conservation of mass, page 34, GCSE Chemistry; The Revision Guide, CGP, OCR Gateway ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Law of conservation of mass, pages 90-91, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 16:28, 13 December 2019
Key Stage 3
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
- In dissolving conservation of mass means that the mass of the solvent and the mass of the solute can be added to find the mass of the solution.
| Masssolvent + Masssolute = Masssolution |
- In a chemical reaction conservation of mass means that the same atoms which made up the reactants must make up the products. So the atoms are not created or destroyed in a chemical reaction, they are just rearranged.
|
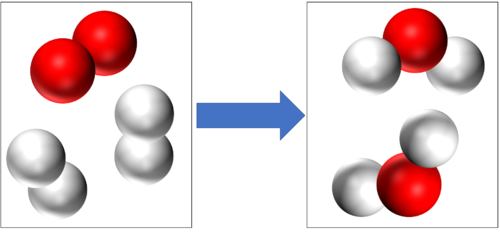
Conservation of mass tells us that if there are 4 Hydrogen atoms and 2 Oxygen atoms at the start of this reaction then there will be the end of the reaction 4 Hydrogen atoms and 2 Oxygen atoms at the end of the reaction. |
|
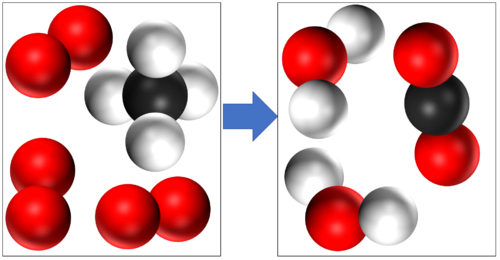
In this reaction you can see that mass is conserved because there are 4 Hydrogen atoms, 4 Oxygen atoms and 1 Carbon atom in the reactants and all the same atoms are found in the products. |
Key Stage 4
Meaning
Conservation of Mass is a law of the universe that states that mass cannot be created or destroyed, it can only be moved from one place to another.
About Conservation of Mass
- In a chemical reaction law of conservation of mass indicates that the total mass of the products is the same as the total mass of the reactants.
- Conservation of mass can be observed in closed systems in which none of the products can escape and no other chemicals enter the system.
- In an open system any chemical reaction which produces a gas will appear to decrease in mass but only because the mass has moved to a different location. The particles of gas escape the container.
Examples
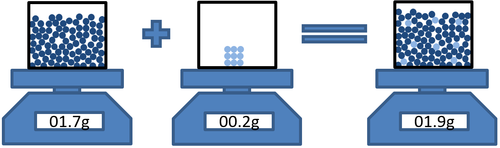
Methane + Oxygen → Water + Carbon Dioxide
CH4 + 2O2 → 2H2O + CO2
16g + 64g = 36g + 44g
Sodium Hydroxide + Hydrochloric Acid → Sodium Chloride + Water
NaOH + HCl → NaCl + H2O
40g + 36.5g = 58.5 + 18g
Calculating the Mass of a missing Product/Reactant
- The mass of a missing product of reactant can be found because the total mass of the products = the total mass of reactants.
MReactants = MProducts
|
Find the mass of Calcium Oxide produced in the following reaction: CaCO3 → CaO + CO2 25g = x + 11g |
Find the mass of Carbon Dioxide produced in the following reaction: CH4 + 2O2 → 2H2O + CO2 4g + 16g = 9g + x |
Find the mass of Hydrochloric Acid needed in the following reaction: NaOH + HCl → NaCl + H2O 160g + x = 234g + 72g |
|
x = 25g - 11g x = 14g |
20g = 9g + x x = 20g - 9g x = 11g |
160g + x = 306g x = 306g - 160g x = 146g |
Calculating the Mass Required for a Complete Reaction
|
Find the mass of Oxygen needed to completely oxidise all of the Magnesium: 2Mg + O2 → 2MgO 48g + x = y |
Find the mass of Oxygen needed for the complete combustion of Methane. CH4 + 2O2 → 2H2O + CO2 32g + x = y |
Find the mass of Hydrochloric Acid needed to completely neutralise all of the Sodium Hydroxide. NaOH + HCl → NaCl + H2O 20g + x = y + z |
|
Find the Relative Formula Mass of the reactants. Mr of Mg = 24g Mr of O2 = 16x2 Mr of O2 = 32g |
Find the Relative Formula Mass of the reactants. Mr of CH4 = 16g Mr of O2 = 16x2 Mr of O2 = 32g |
Find the Relative Formula Mass of the reactants. Mr of NaOH = 40g Mr of HCl = 36.5g |
|
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{48}{24}\) No. Moles = 2 Mole Therefore 1 mole of O2 is needed. 1 mole of O2 = 32g |
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{32}{16}\) No. Moles = 2 Mole Therefore 4 moles of O2 are needed. 4 moles of O2 = 128g |
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{20}{40}\) No. Moles = 0.5 Mole Therefore 0.5 mole of HCl is needed. 0.5 mole of HCl = 18.25g |
Beyond the Curriculum
References
AQA
- Conservation of mass, page 181, GCSE Combined Science Trilogy 1, Hodder, AQA
- Conservation of mass, page 43, GCSE Chemistry; The Revision Guide, CGP, AQA
- Conservation of mass, page 6, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Conservation of mass, page 68, GCSE Chemistry, Hodder, AQA
- Conservation of mass, page 78, GCSE Physics; Third Edition, Oxford University Press, AQA
- Conservation of mass, pages 108-110, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Conservation of mass, pages 114- 116, GCSE Chemistry, CGP, AQA
- Conservation of mass, pages 124, 125, 195, GCSE Combined Science; The Revision Guide, CGP, AQA
- Conservation; of mass, pages 88, 116-17, GCSE Physics; Student Book, Collins, AQA
Edexcel
- Conservation of mass, apge 26, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Conservation of mass, law of, pages 218-219, GCSE Combined Science, Pearson Edexcel
- Conservation of mass, law of, pages 74-75, GCSE Chemistry, Pearson, Edexcel
- Conservation of mass, page 89, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Conservation of mass, pages 74-76, GCSE Chemistry, CGP, Edexcel
- Mass conservation, pages 74-75, GCSE Chemistry, Pearson, Edexcel