Difference between revisions of "Extracting Metals by Electrolysis"
(Created page with "==Key Stage 3== ===Meaning=== A '''electrolysis''' is a way to extract metals from minerals when the metal is more Reactivity Series|reactiv...") |
|||
| Line 9: | Line 9: | ||
|[[File:ElectrolysisCell.png|center|500px]] | |[[File:ElectrolysisCell.png|center|500px]] | ||
|- | |- | ||
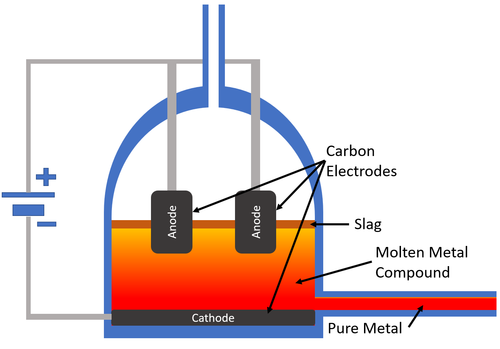
| − | | style="height:20px; width:200px; text-align:center;" |[[Carbon]] [[electrode]]s are used to pass an [[Electrical Current]] through the [[molten]] [[mineral]]. The [[metal]] collects at the [[cathode]] which carries a negative [[charge]] and the [[pure]] [[metal]] is removed from the [[Electrolysis Cell|electrolysis cell]]. | + | | style="height:20px; width:200px; text-align:center;" |[[Carbon]] [[electrode]]s are used to pass an [[Electrical Current]] through the [[molten]] [[mineral]]. The [[metal]] collects at the [[cathode]] which carries a negative [[Electrical Charge|charge]] and the [[pure]] [[metal]] is removed from the [[Electrolysis Cell|electrolysis cell]]. |
|} | |} | ||
Revision as of 14:01, 31 October 2018
Key Stage 3
Meaning
A electrolysis is a way to extract metals from minerals when the metal is more reactive than Carbon.
About Extracting Metals by Electrolysis
- Metals that are more reactive than Carbon cannot be displaced with Carbon so electrolysis is used to extract them.
| Carbon electrodes are used to pass an Electrical Current through the molten mineral. The metal collects at the cathode which carries a negative charge and the pure metal is removed from the electrolysis cell. |