Difference between revisions of "Ice-Water Anomaly"
(→Key Stage 3) |
|||
| Line 11: | Line 11: | ||
|[[File:IceWaterAnomaly.png|center|500px]] | |[[File:IceWaterAnomaly.png|center|500px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" |The same number of [[molecule]]s takes up a larger [[volume]] in [[solid]] [[water]], so [[Ice]] is less [[Density|dense]] than [[liquid]] [[water]]. | + | | style="height:20px; width:200px; text-align:center;" |The same number of [[molecule]]s takes up a larger [[Volume (Space)|volume]] in [[solid]] [[water]], so [[Ice]] is less [[Density|dense]] than [[liquid]] [[water]]. |
|} | |} | ||
Revision as of 11:39, 1 November 2018
Key Stage 3
Meaning
The Ice-Water Anomaly is the observation that water in its solid state is less dense than water in its liquid state.
About the Ice-Water Anomaly
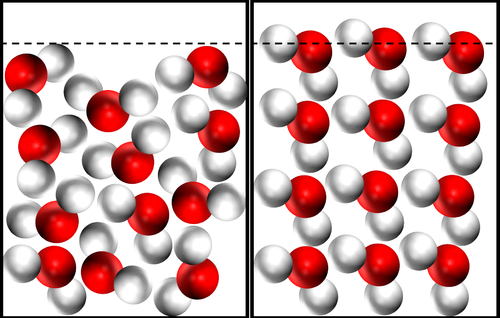
- For most substances the solid state is more dense than the liquid state. However, this is not the case for water.
- In liquid water the molecules are randomly arranged and close together. In solid water the molecules align with their Hydrogen atoms touching the Oxygen atoms of adjacent molecules.
| The same number of molecules takes up a larger volume in solid water, so Ice is less dense than liquid water. |