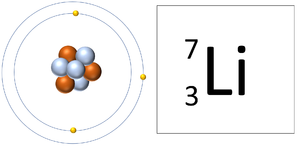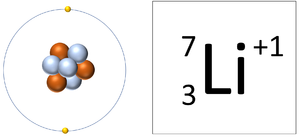Difference between revisions of "Ion"
(→About Ions) |
|||
| Line 23: | Line 23: | ||
|- | |- | ||
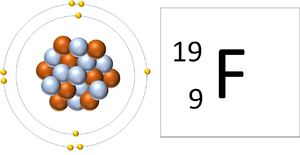
| style="height:20px; width:300px; text-align:center;" |A [[Fluorine]] [[atom]] has 9 [[proton]]s and 9 [[electron]]s so it is [[Neutral Charge|neutral]]. | | style="height:20px; width:300px; text-align:center;" |A [[Fluorine]] [[atom]] has 9 [[proton]]s and 9 [[electron]]s so it is [[Neutral Charge|neutral]]. | ||
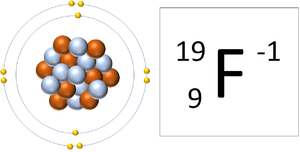
| − | | style="height:20px; width:300px; text-align:center;" |A [[Fluorine]] [[ | + | | style="height:20px; width:300px; text-align:center;" |A [[Fluorine]] [[ion]] has 9 [[proton]]s and 10 [[electron]]s so it is [[Negative Charge|negative charge]]. It now has a full [[Outer Shell]]. |
|} | |} | ||
Revision as of 21:12, 25 November 2018
Key Stage 4
Meaning
An Ion is a particle that has a different number of protons to electrons.
About Ions
- An atom contains the same number of protons as electrons. When an atom loses or gains electrons it becomes an ion.
- Ions can be positively charged or negatively charged.
- If an atom gains electrons it becomes a negatively charged ion since there are more electrons than protons and electrons carry a negative charge.
- If an atom loses electrons it becomes a positively charged ion since there are more protons than electrons and protons carry a positive charge.
| Atom | Ion |
| A Lithium atom has 3 protons and 3 electrons so it is neutral. | A Lithium ion has 3 protons and 2 electrons so it has a positive charge. It now has a full Outer Shell. |
| A Fluorine atom has 9 protons and 9 electrons so it is neutral. | A Fluorine ion has 9 protons and 10 electrons so it is negative charge. It now has a full Outer Shell. |