Difference between revisions of "Corrosion"
(→Examples) |
(→Preventing and Reducing Corrosion) |
||
| Line 30: | Line 30: | ||
===Preventing and Reducing Corrosion=== | ===Preventing and Reducing Corrosion=== | ||
| + | : [[Corrosion]] can be prevented by making a physical barrier between the [[object]] and the [[chemical]]s in the environment. This can be done by painting or [[electroplating]]. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:RustingPipe.png|center|200px]] | ||
| + | |[[File:Electroplating.png|center|200px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |This [[Iron]] pipe was painted to prevent [[corrosion]], but where the paint has chipped away, the [[Iron]] has [[rusting|rusted]]. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Electroplating]] can cover a [[Reactivity Series|reactive]] [[metal]] with a less [[Reactivity Series|reactive]] [[metal]] such as [[Copper]] create a physical barrier between the [[object]] and the environment. | ||
| + | |} | ||
| + | |||
| + | : [[Corrosion]] of a [[metal]] [[object]] can also be prevented using a [[Sacrificial Anode]] which is a [[metal]] more [[Reactivity Series|reactive]] [[metal]] placed in [[Electrical Contact|electrical contact]] with the [[object]]. This prevents [[Oxidation]] of the [[object]] | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:SacrificialAnode.png|center|300px]] | ||
| + | |- | ||
| + | | style="height:20px; width:300px; text-align:center;" |A [[Sacrifical Anode]] made of [[Zinc]] has been attached to the [[Iron]] hull of a boat. | ||
| + | |} | ||
Revision as of 17:05, 26 January 2019
Contents
Key Stage 3
Meaning
Corrosion is when a material is worn down by chemical reactions with substances in the environment.
About Corrosion
- Rusting is a form of corrosion.
- Acid rain causes the corrosion of limestone and marble buildings and statues.
Key Stage 4
Meaning
Corrosion is when a material is worn down by chemical reactions with substances in the environment.
About Corrosion
- Corrosion is commonly caused by Water, Oxygen and Acids in the environment.
- Metals and stone (such as limestone, marble and chalk) are the most commonly corroded materials.
- More reactive metals are more easily corroded.
Examples
| The corrosion of Iron forms brown Iron Oxide, known as rust. Rusting happens more quickly in humid air or in salty water. | The corrosion of Copper forms green Copper Carbonate. Initially the Copper is Oxidised to form Copper Oxide, a black solid, but then reacts with Carbonic Acid to form Copper Carbonate. | This statue is made of Limestone which is mostly Calcium Carbonate. It is corroded by Acid Rain to form soluble salts which wash away. |
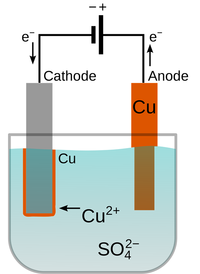
Preventing and Reducing Corrosion
- Corrosion can be prevented by making a physical barrier between the object and the chemicals in the environment. This can be done by painting or electroplating.
| This Iron pipe was painted to prevent corrosion, but where the paint has chipped away, the Iron has rusted. | Electroplating can cover a reactive metal with a less reactive metal such as Copper create a physical barrier between the object and the environment. |
- Corrosion of a metal object can also be prevented using a Sacrificial Anode which is a metal more reactive metal placed in electrical contact with the object. This prevents Oxidation of the object
| A Sacrifical Anode made of Zinc has been attached to the Iron hull of a boat. |