Difference between revisions of "Methanoic Acid"
| (2 intermediate revisions by one other user not shown) | |||
| Line 31: | Line 31: | ||
[[Methanoic Acid]] can be [[neutralise (Chemistry)|neutralised]] to [[Product|produce]] [[Organic Compound|organic]] [[salt]]s. | [[Methanoic Acid]] can be [[neutralise (Chemistry)|neutralised]] to [[Product|produce]] [[Organic Compound|organic]] [[salt]]s. | ||
: [[Methanoic Acid]] + [[Sodium]] → [[Sodium Methanoate]] + [[Hydrogen]] | : [[Methanoic Acid]] + [[Sodium]] → [[Sodium Methanoate]] + [[Hydrogen]] | ||
| − | + | ||
| + | <math>2HCOOH + 2Na → 2HCOONa + H_2</math> | ||
: [[Methanoic Acid]] + [[Potassium Oxide]] → [[Potassium Methanoate]] + [[Water]] | : [[Methanoic Acid]] + [[Potassium Oxide]] → [[Potassium Methanoate]] + [[Water]] | ||
| − | + | ||
| + | <math>2HCOOH + 2K → 2HCOOK + H_2O</math> | ||
: [[Methanoic Acid]] + [[Lithium Hydroxide]] → [[Lithium Methanoate]] + [[Water]] | : [[Methanoic Acid]] + [[Lithium Hydroxide]] → [[Lithium Methanoate]] + [[Water]] | ||
| − | + | ||
| + | <math>HCOOH + LiOH → HCOOLi + H_2O</math> | ||
: [[Methanoic Acid]] + [[Magnesium Carbonate]] → [[Magnesium Methanoate]] + [[Carbon Dioxide]] + [[Water]] | : [[Methanoic Acid]] + [[Magnesium Carbonate]] → [[Magnesium Methanoate]] + [[Carbon Dioxide]] + [[Water]] | ||
| − | + | ||
| + | <math>2HCOOH + MgCO_3 → (HCOO)_2Mg + CO_2 + H_2O</math> | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Methanoic acid, page 242, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Methanoic acid, pages 244-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
Latest revision as of 15:47, 8 November 2019
Contents
Key Stage 3
Meaning
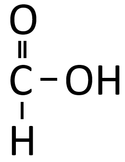
Methanoic Acid is a compound with chemical formula CH2O2.
About Methanoic Acid
- Methanoic Acid is a solid dissolved in water to form a solution.
Methanoic Acid can be neutralised to produce organic salts.
- Methanoic Acid + Sodium → Sodium Methanoate + Hydrogen
- Methanoic Acid + Potassium Oxide → Potassium Methanoate + Water
- Methanoic Acid + Lithium Hydroxide → Lithium Methanoate + Water
- Methanoic Acid + Magnesium Carbonate → Magnesium Methanoate + Carbon Dioxide + Water
Key Stage 4
Meaning
Methanoic Acid is a Carboxylic Acid with chemical formula CH2O2.
About Methanoic Acid
- Methanoic Acid is an aqueous solution.
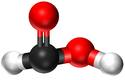
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| CH2O2 | HCOOH |
Methanoic Acid can be neutralised to produce organic salts.
\(2HCOOH + 2Na → 2HCOONa + H_2\)
\(2HCOOH + 2K → 2HCOOK + H_2O\)
\(HCOOH + LiOH → HCOOLi + H_2O\)
\(2HCOOH + MgCO_3 → (HCOO)_2Mg + CO_2 + H_2O\)