Difference between revisions of "Evaporation of Solutions"
(Created page with "==Key Stage 2== ===Meaning=== '''Evaporation of a solution''' is a way to get back a solid that has been dissolved in a liquid. ===About Evaporation of Solutions=...") |
(→About Evaporation of Solutions) |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
===About Evaporation of Solutions=== | ===About Evaporation of Solutions=== | ||
| − | : When you heat a [[solution]] the [[liquid]] will [[evaporate]] leaving the solid behind. | + | : When you heat a [[solution]] the [[liquid]] will [[evaporating|evaporate]] leaving the [[solid]] behind. |
{| class="wikitable" | {| class="wikitable" | ||
| Line 11: | Line 11: | ||
|[[File:EvaporatingDish.png|center|200px]] | |[[File:EvaporatingDish.png|center|200px]] | ||
|} | |} | ||
| + | |||
| + | ===Examples=== | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |[[File:SaltEvaporationPonds.png|center|400px]] | ||
| + | |- | ||
| + | | style="height:20px; width:400px; text-align:center;" |These people collect salt by putting sea water in small ponds and allowing the warm temperatures to evaporate the water away leaving the behind the salt. | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 3== | ||
| + | ===Meaning=== | ||
| + | '''Evaporation of a solution''' is a way to [[Separating Mixtures|separate]] the [[mixture]] of a [[solution]] to recover the [[solute]] that has been [[dissolve]]d in a [[solvent]]. | ||
| + | |||
| + | ===About Evaporation of Solutions=== | ||
| + | : The '''evaporation of solutions''' recovers the [[solute]]s but loses the [[solvent]]. | ||
| + | : '''Evaporation of solutions''' can be done by directly heating the [[solution]] or by giving time for the [[liquid]] to [[evaporating|evaporate]] at low [[temperature]]s. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
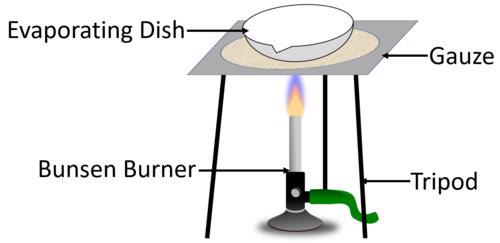
| + | |[[File:EvaporatingDiagram.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |The [[diagram]] shows the experimental setup to [[evaporating|evaporate]] away the [[solvent]] to leave behind the [[solute]] in the [[Evaporating Dish|evaporating dish]]. | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | '''Evaporation of solution''', sometimes called '''Crystallisation''', is a technique used to [[Separating Mixtures|separate]] the [[solute]] from the [[solvent]] in a [[solution]], losing the [[solvent]]. | ||
| + | |||
| + | ===About Evaporation of Solutions=== | ||
| + | '''Evaporation of solutions''' can only be used for: | ||
| + | *[[Separating Mixtures|Separating]] a [[solution]] to recover [[solute]]s from the [[solution]]s. | ||
| + | *[[Separating Mixtures|Separating]] [[insoluble]] [[solid]]s from a [[liquid]]. However, this is unnecessary as it can be done by [[filtration]]. | ||
| + | '''Evaporation of solutions''' cannot be used for: | ||
| + | *Recovering the [[solvent]] from a [[solution]] - [[Distillation]] | ||
| + | *[[Separating Mixtures|Separating]] two [[solute]]s from each other in [[solution]] - [[Chromatography]] | ||
| + | *[[Separating Mixtures|Separating]] an [[insoluble]] [[solid]] from a [[soluble]] [[solid]] - [[Filtration]] | ||
| + | *[[Separating Mixtures|Separating]] two [[solvent]]s from each other in [[solution]] - [[Fractional Distillation]] | ||
Latest revision as of 10:36, 21 January 2019
Contents
Key Stage 2
Meaning
Evaporation of a solution is a way to get back a solid that has been dissolved in a liquid.
About Evaporation of Solutions
| You can separate salt from the water by evaporating the water in an evaporating dish. |
Examples
| These people collect salt by putting sea water in small ponds and allowing the warm temperatures to evaporate the water away leaving the behind the salt. |
Key Stage 3
Meaning
Evaporation of a solution is a way to separate the mixture of a solution to recover the solute that has been dissolved in a solvent.
About Evaporation of Solutions
- The evaporation of solutions recovers the solutes but loses the solvent.
- Evaporation of solutions can be done by directly heating the solution or by giving time for the liquid to evaporate at low temperatures.
| The diagram shows the experimental setup to evaporate away the solvent to leave behind the solute in the evaporating dish. |
Key Stage 4
Meaning
Evaporation of solution, sometimes called Crystallisation, is a technique used to separate the solute from the solvent in a solution, losing the solvent.
About Evaporation of Solutions
Evaporation of solutions can only be used for:
- Separating a solution to recover solutes from the solutions.
- Separating insoluble solids from a liquid. However, this is unnecessary as it can be done by filtration.
Evaporation of solutions cannot be used for:
- Recovering the solvent from a solution - Distillation
- Separating two solutes from each other in solution - Chromatography
- Separating an insoluble solid from a soluble solid - Filtration
- Separating two solvents from each other in solution - Fractional Distillation