Difference between revisions of "Corrosion"
| Line 51: | Line 51: | ||
| style="height:20px; width:300px; text-align:center;" |A [[Sacrificial Anode]] made of [[Zinc]] has been attached to the [[Iron]] hull of a boat. | | style="height:20px; width:300px; text-align:center;" |A [[Sacrificial Anode]] made of [[Zinc]] has been attached to the [[Iron]] hull of a boat. | ||
|} | |} | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Corrosion, page 338-9, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d94 ''Corrosion, page 98, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Corrosion, pages 220-221, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Corrosion, pages 258-9, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Corrosion, pages 287, 288, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Corrosion; prevention of, pages 259-60, GCSE Chemistry, Hodder, AQA ''] | ||
Revision as of 22:57, 3 November 2019
Contents
Key Stage 3
Meaning
Corrosion is when a material is worn down by chemical reactions with substances in the environment.
About Corrosion
- Rusting is a form of corrosion.
- Acid rain causes the corrosion of limestone and marble buildings and statues.
Key Stage 4
Meaning
Corrosion is when a material is worn down by chemical reactions with substances in the environment.
About Corrosion
- Corrosion is commonly caused by Water, Oxygen and Acids in the environment.
- Metals and stone (such as limestone, marble and chalk) are the most commonly corroded materials.
- More reactive metals are more easily corroded.
Examples
| The corrosion of Iron forms brown Iron Oxide, known as rust. Rusting happens more quickly in humid air or in salty water. | The corrosion of Copper forms green Copper Carbonate. Initially the Copper is Oxidised to form Copper Oxide, a black solid, but then reacts with Carbonic Acid to form Copper Carbonate. | This statue is made of Limestone which is mostly Calcium Carbonate. It is corroded by Acid Rain to form soluble salts which wash away. |
Preventing and Reducing Corrosion
- Corrosion can be prevented by making a physical barrier between the object and the chemicals in the environment. This can be done by painting, covering something in grease or by electroplating.
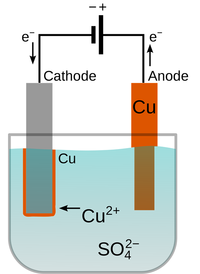
| This Iron pipe was painted to prevent corrosion, but where the paint has chipped away, the Iron has rusted. | Electroplating can cover a reactive metal with a less reactive metal such as Copper create a physical barrier between the object and the environment. | Grease is used on bike chains to prevent corrosion. Paint and electroplating cannot be used because they would chip away from constant movement of the chain. |
- Corrosion of a metal object can also be prevented using a Sacrificial Anode which is a metal more reactive metal placed in electrical contact with the object. This prevents Oxidation of the object because the more reactive metal has a greater tendency to form positive ions causing it to oxidise instead of the object.
| A Sacrificial Anode made of Zinc has been attached to the Iron hull of a boat. |
References
AQA
- Corrosion, page 338-9, GCSE Chemistry; Student Book, Collins, AQA
- Corrosion, page 98, GCSE Chemistry; The Revision Guide, CGP, AQA
- Corrosion, pages 220-221, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Corrosion, pages 258-9, GCSE Chemistry, Hodder, AQA
- Corrosion, pages 287, 288, GCSE Chemistry, CGP, AQA
- Corrosion; prevention of, pages 259-60, GCSE Chemistry, Hodder, AQA