Difference between revisions of "Catalyst"
(→About Catalysts) |
|||
| Line 19: | Line 19: | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| − | |[[File: | + | |[[File:ExothermicSketchGraphWithoutCatalyst.png|center|300px]] |
| − | |[[File: | + | |[[File:ExothermicSketchGraphWithCatalyst.png|center|300px]] |
|- | |- | ||
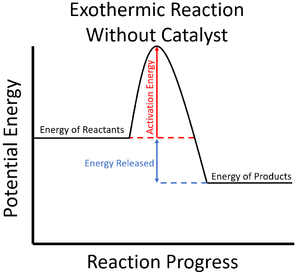
| style="height:20px; width:200px; text-align:center;" |The [[Activation Energy|activation energy]] is shown to be high in this [[Reaction Profile|reaction profile]] for an [[exothermic]] [[Chemical Reaction|reaction]] without a [[catalyst]]. | | style="height:20px; width:200px; text-align:center;" |The [[Activation Energy|activation energy]] is shown to be high in this [[Reaction Profile|reaction profile]] for an [[exothermic]] [[Chemical Reaction|reaction]] without a [[catalyst]]. | ||
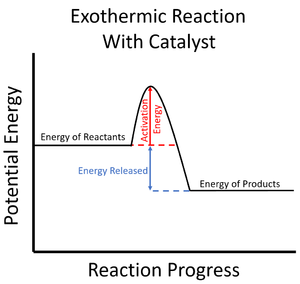
| style="height:20px; width:200px; text-align:center;" |The [[Activation Energy|activation energy]] is shown to be low in this [[Reaction Profile|reaction profile]] for an [[exothermic]] [[Chemical Reaction|reaction]] with a [[catalyst]]. | | style="height:20px; width:200px; text-align:center;" |The [[Activation Energy|activation energy]] is shown to be low in this [[Reaction Profile|reaction profile]] for an [[exothermic]] [[Chemical Reaction|reaction]] with a [[catalyst]]. | ||
|} | |} | ||
Revision as of 14:20, 16 January 2019
Contents
Key Stage 3
Meaning
A catalyst is a chemical which can increase the rate of a chemical reaction without being used up in the reaction.
About Catalysts
- A catalyst is neither are reactant nor a product in a reaction but it can make the reaction happen more quickly.
Examples
- Chlorophyll is a catalyst for the reaction that allows plants to turn Carbon Dioxide and Water into Glucose and Oxygen during photosynthesis.
- Manganese Dioxide is a catalyst that speeds up the break down of Hydrogen Peroxide into Water and Oxygen.
- Enzymes are a type of biological catalyst that can speed up reaction inside an organism.
Key Stage 4
Meaning
A catalyst is a chemical which can increase the rate of reaction and lower the activation energy for a particular reaction.
About Catalysts
| The activation energy is shown to be high in this reaction profile for an exothermic reaction without a catalyst. | The activation energy is shown to be low in this reaction profile for an exothermic reaction with a catalyst. |